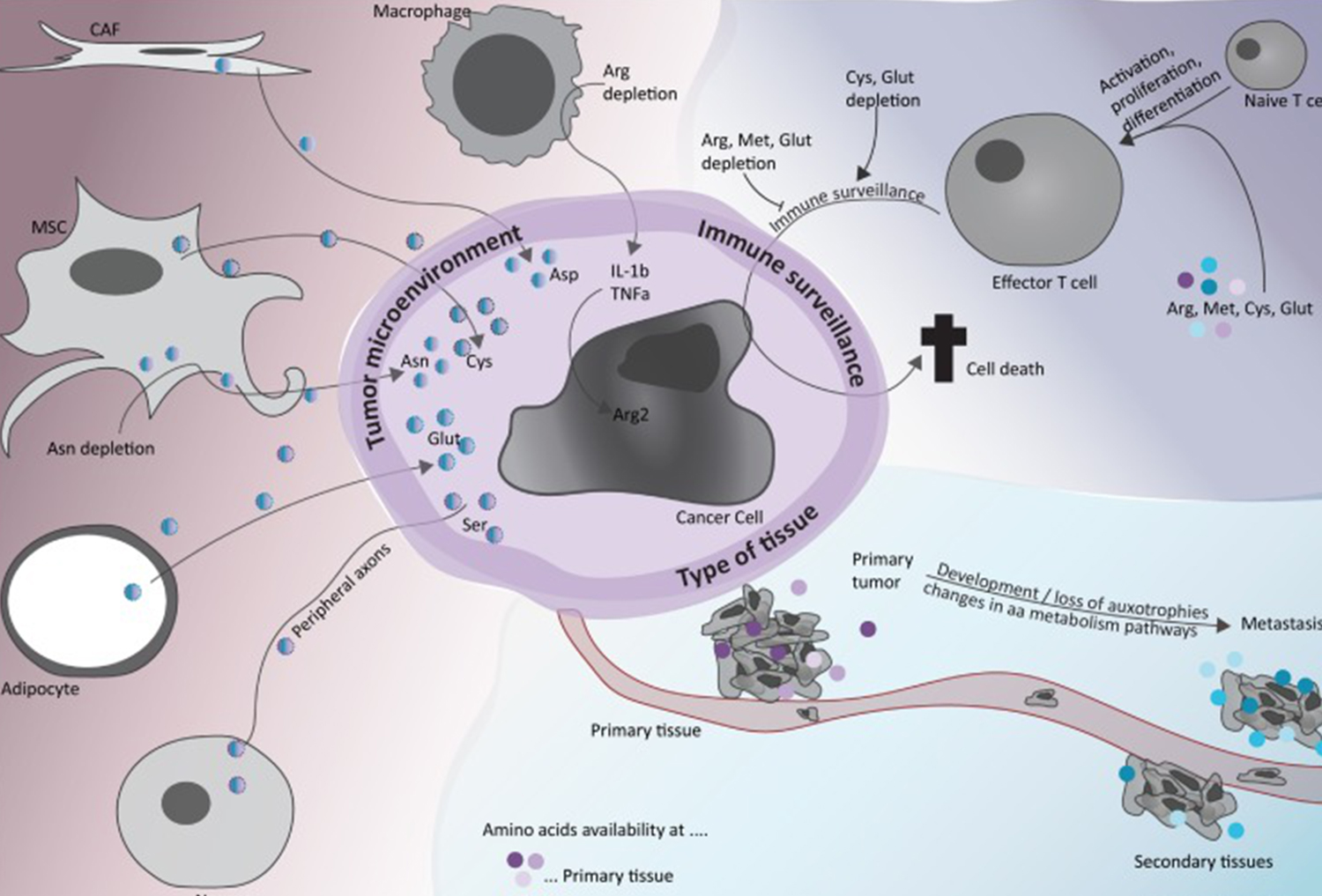
Anti-tumor targeted drugs refer to anti-tumor drugs that selectively interfere with the excessive proliferation, infiltration and metastasis of tumor cells by targeting the key regulatory molecules in the pathological process of tumor molecules. They often only act on tumor cells, but do not cause significant damage to normal cells. According to the targets and properties of drugs, they can usually be divided into monoclonal antibodies and small molecule inhibitors.
Monoclonal antibody drugs: take the growth factor or extracellular receptor tyrosine kinase free in the blood as the target, such as B lymphocyte antigen CD20, human epidermal growth factor receptor 2 (HER2), vascular endothelial growth factor (VEGF), epidermal growth factor receptor (EGFR), etc., and represent the drugs such as rituximab, trastuzumab, bevacizumab, patuzumab, etc.
Small molecule targeted drugs: take key kinases in the cell signal transduction pathway as targets, such as EGFR, vascular endothelial growth factor receptor (VEGFR), bcr-abl fusion gene, anaplastic lymphoma kinase (ALK), rapidly accelerating fibrosarcoma (RAF), mitogen activated protein kinase (MEK), mammalian rapamycin target protein (mTOR), etc. the representative drugs include gefitinib, sorafenib, imatinib, trimettinib Everolimus et al.
1. Application principles of anti-tumor targeted drugs
It can be used only after confirmed by histopathology;
It can only be used after target detection (Table 1);
Strictly follow the indications;
Reflect the treatment value of patients;
Rational use of drugs under special circumstances;
Pay attention to drug-related adverse reactions.
Table 1 requirements for target detection of common targeted drugs (the following table is derived from reference [1])
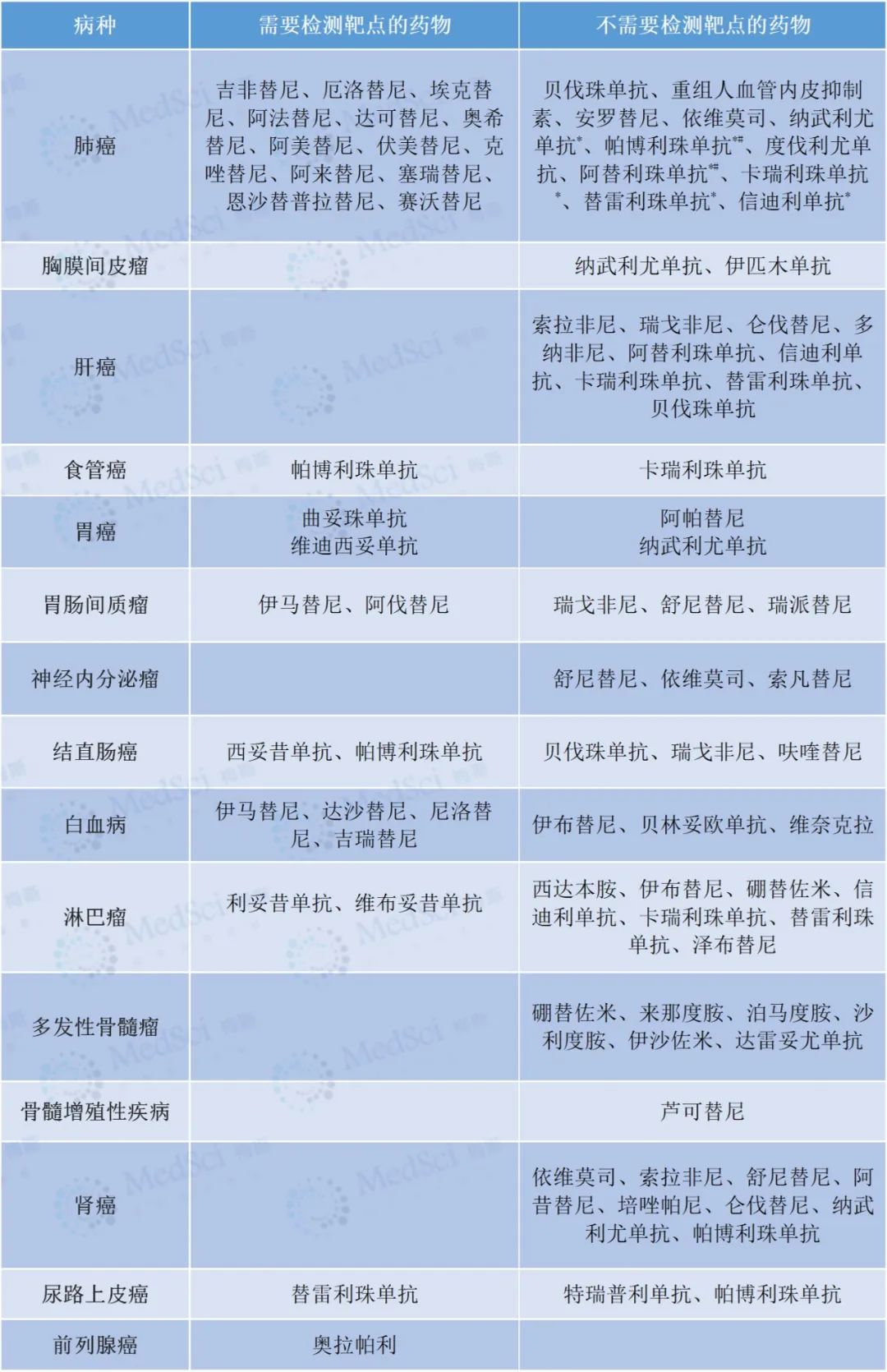
*: Patients with EGFR gene mutation and alk fusion positive should be excluded.
#: PD-L1 expression should be detected before the use of pabolizumab and atelizumab alone.
2. Indications, usage and precautions for first-line treatment of common anti-tumor targeted drugs
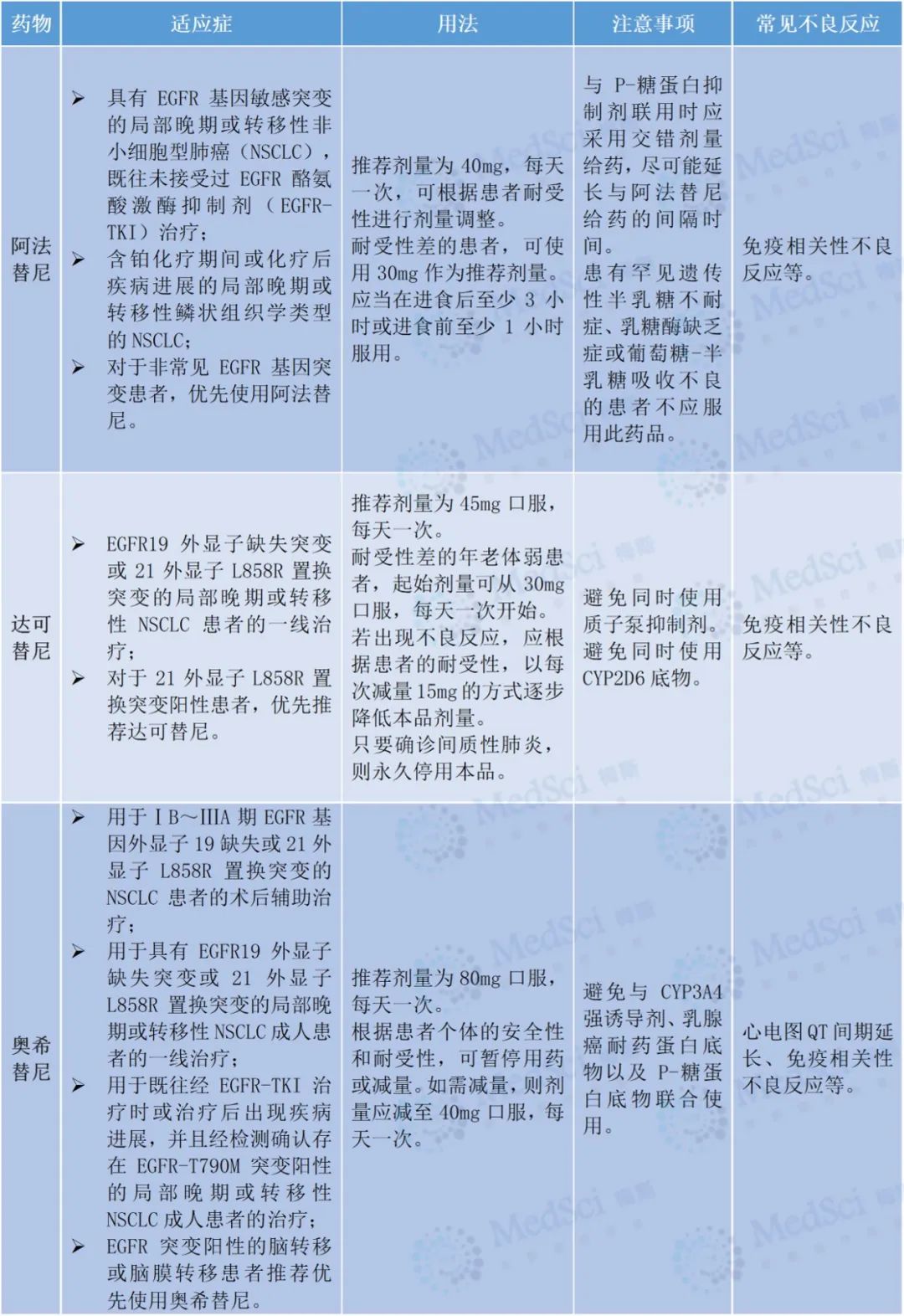
3. Adverse reaction treatment adjustment scheme
(1) Immune related adverse reactions and treatment adjustment protocol (the following table is derived from reference [1])

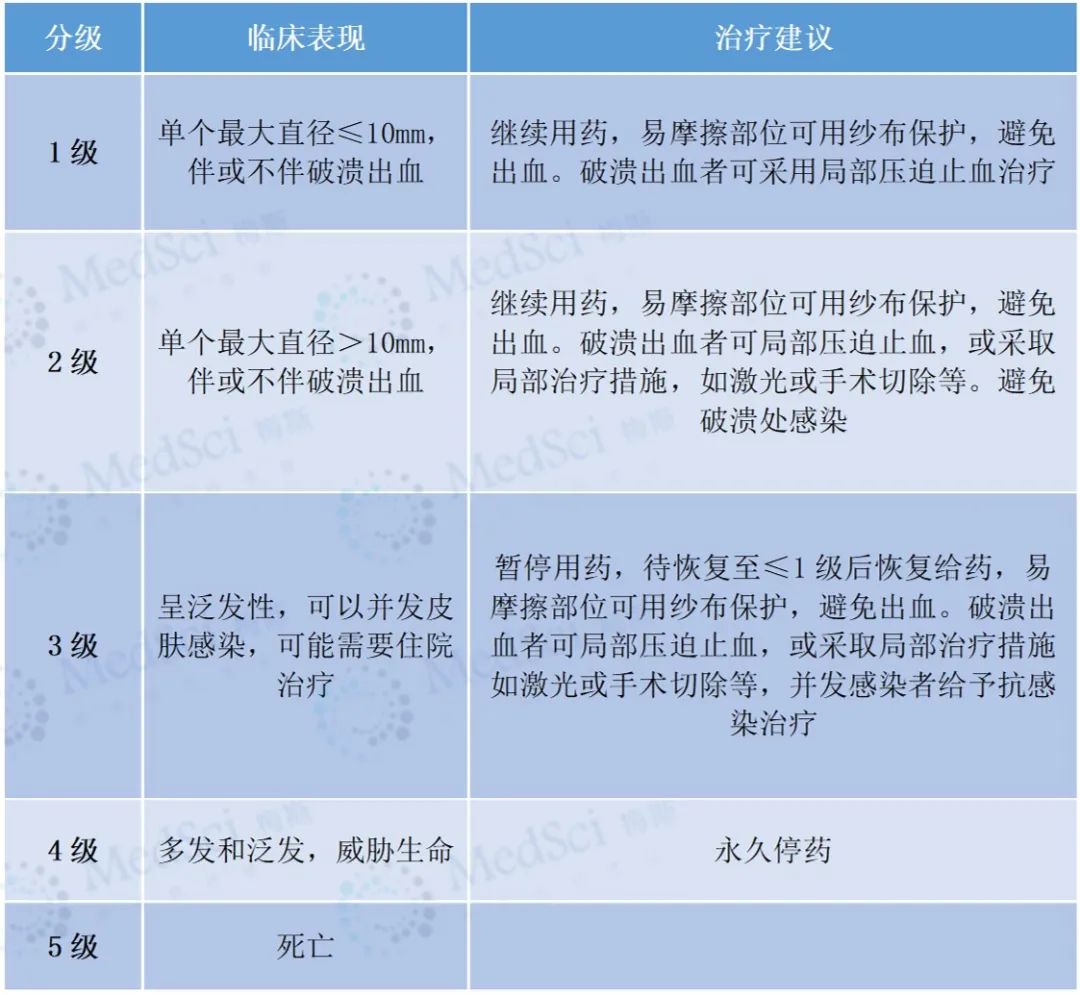
(2) Grading criteria and treatment recommendations for reactive capillary hyperplasia (the following table is derived from reference [1])
4. Analysis of common clinical problems
(1) How to choose the time of taking oral targeted drugs?
Answer: the time of taking oral targeted drugs includes taking drugs on an empty stomach (1 hour before or 2 hours after a meal), taking drugs before a meal (15-30 minutes before a meal), taking drugs during a meal (taking drugs after a little meal, and then continuing to eat), taking drugs after a meal (taking drugs 15-30 minutes after a meal), taking drugs in the morning (taking drugs in the morning), and taking drugs before going to bed (taking drugs 15-30 minutes before going to bed). Considering that the metabolism of targeted drugs may be affected by food or gastric acid, and may cause related adverse reactions (such as gastrointestinal reactions, etc.), patients should be advised to refer to the drug instructions for specific medication time.
(2) If vomiting occurs or the drug is missed once during the course of taking the drug, do you need to take it again or in the next prescription dose?
A: in case of missed dose, and the time interval between the next medication is more than 12 hours, you can supplement the missed dose; If the interval between the next time of taking medicine is less than 12 hours, it may not be taken again. In case of vomiting, it is not recommended to take additional medicine. The next time of taking medicine shall be the normal dose. Refer to the drug instructions for specific requirements.
reference:
[1] Guangdong Pharmaceutical Association Expert consensus on clinical application and adverse reaction management of platinum drugs [j] Pharmacy today, 2019, 29 (9): 361-369
[2] Drug description
Article source: