Akirensai injection, the first anticancer drug of car-t therapy approved in China on June 22 this year, is the first user in China. According to recent media reports, it has gone through the discharge formalities from Shanghai Ruijin Hospital on August 26. What is surprising is that after car-t treatment, the patient with diffuse large B-cell lymphoma (DLBCL), After evaluation, the criteria for complete mitigation have been met.
In the field of cancer treatment, we usually don't talk about "cure". The word "remission" is generally used to evaluate the treatment of malignant tumors. When the cancer signs and symptoms decrease or disappear, we call it "remission", while complete remission refers to the complete disappearance of the patient's relevant cancer symptoms. In a sense, it also achieves a "cure" The effect of.
This injection is so magical that it can cure cancer patients and let many cancer patients see more hope. At present, the price of this drug is 1.2 million a shot. Such a high price makes many people sigh. What is this car-t therapy? Can it cure all kinds of cancer problems? Let's talk about this topic with you today.
What is car-t therapy?
Although akirensai injection is a drug, in fact, it is mainly used for car-t therapy. The full name of this therapy is chimeric antigen receptor T cell immunotherapy. It is a new precision targeted therapy in recent years. With the continuous progress of medical level, this therapy is considered to be a cellular immunotherapy that is "possible to cure cancer".
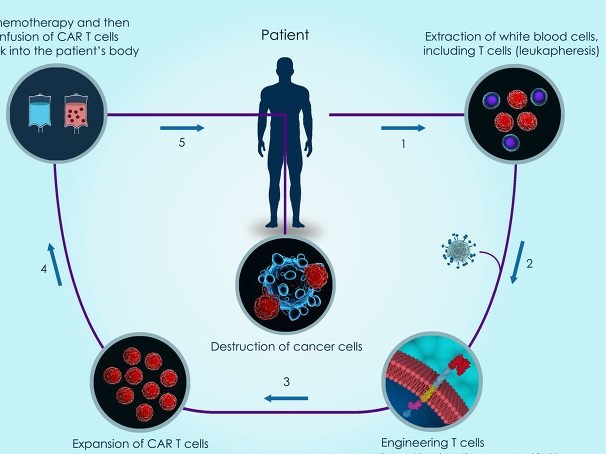
T in car-t therapy refers to T lymphocytes, which are a kind of white blood cells in the human body. As we all know, white blood cells are important cells that play an important role in human immunity, while T cells are important immune cells that play an important role in human blood, lymph and surrounding tissues and organs. These cells can play an immune role in diseases such as bacterial infection and tumor.
However, it is difficult to eliminate cancer cells only by T lymphocytes. Car in car-t therapy is called tumor signature antigen receptor. Through genetic engineering technology, we form a community between T cells isolated from human body and car, which is equivalent to equipping T cells with a "sight" that can accurately identify tumor cells, Through the autoimmune action of human T cells, a large number of anti-cancer factors are released. These anti-cancer factors can kill tumor cells efficiently and accurately, so as to achieve the purpose of treating malignant tumors.
If we compare T cells to anti-cancer guards in the body, car-t therapy adds a special "anti-tumor weapon" to our guards in the body through genetic engineering transformation. This weapon has guidance function, so as to achieve the role of accurately eliminating tumor cells.
Why are car-t drugs so expensive?
Many friends can't understand why a new drug has good curative effect on cancer, but the price makes ordinary people can't afford it at all? In fact, the pricing of this drug is usually considered from many aspects. On the one hand, it is the R & D investment in drug development. Generally speaking, the R & D investment of completely newly developed drugs is more than 100 million yuan. In order to recover the cost, drug enterprises often set a high price when drugs are listed.
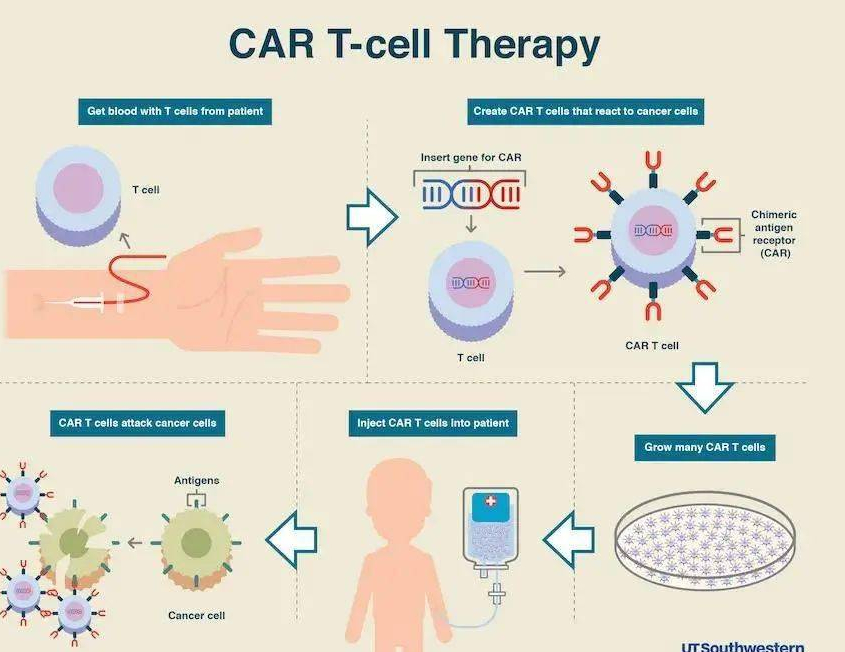
Another reason why this drug is priced at 1.2 million is that it is based on car-t therapy. From the above introduction, we can see that car-t therapy is a treatment method, not a process that may take effect only by injecting the drug solution into the body. For car-t therapy, T cells need to be isolated from cancer patients first, And carry out gene transformation and culture in vitro. Finally, it needs to be expanded to billions or even tens of billions of car-t cells, and then these cultured cells can be reinfused into the patient's body before they can play their anti-cancer role. In other words, the use of this drug needs to be customized in combination with the patient, and it also needs to go through complex technical procedures and processes, Therefore, as a drug with complex therapy, its price is higher than other drugs used only.
Compared with other similar products on the market, the price of this drug is not too high. Kymriah injection approved in the United States in 2017 is mainly used for adolescent and child patients. It has good effects on recurrent, refractory and adolescent B-cell acute lymphoblastic leukemia. Its price is $475000, while abecma injection approved for marketing in March 2021, Priced at US $420000, it is used to treat patients with recurrent and refractory multiple myeloma after four or more advanced therapies.
1.2 million Can the anti-cancer drug with a needle really cure cancer?
Any drug has its indications, and even the most expensive drugs can not cure all diseases. The approved indications of akirensai injection are: for second-line and above systematic treatment of adult patients with relapse and large B-cell lymphoma. The first patient who has achieved complete remission and discharged from hospital mentioned above is the symptomatic patient using this drug and therapy. In fact, we should see the following key points for the scope of use of this drug: first, the patients who have been treated with conventional system with poor effect, second, only for large B-cell lymphoma, and third, adult patients, Only when these three conditions are met, it is possible to obtain definite curative effect by using this drug. The high R & D cost and narrow scope of application are also one of the main reasons for the high pricing of such drugs.
From the current research and development direction of car-t, the therapeutic drugs and methods in this regard are mainly aimed at patients with non solid tumors. They usually have a certain range of applications and good curative effects in patients with malignant blood diseases, lymphoma and multiple myeloma. For some common solid tumors such as gastric cancer, liver cancer, colorectal cancer and other diseases, At present, such drugs can not play a therapeutic role. At present, the treatment of solid tumors by immune cell therapy is still in the experimental stage. We also hope that our scientific researchers can make a breakthrough in the cellular immunotherapy of these more common cancers, so that more cancer patients can benefit.
Cancer is a malignant disease in which normal cells in the body mutate, resulting in abnormal proliferation of mutated cells, resulting in canceration, affecting health and even life-threatening. So far, there are few therapies and drugs that can completely alleviate cancer, and people's research and development of drugs and methods for cancer treatment has never stopped, But for us personally, if we can minimize exposure to various carcinogenic factors in our daily life, such as eating less junk food containing carcinogens, staying up late, quitting smoking and limiting alcohol, taking active exercise, maintaining good health and immunity, we can greatly reduce our risk of cancer, Only by doing a good job in cancer prevention and anti-cancer from the perspective of basic life is the basis for reducing the occurrence and development of cancer, which is worthy of our persistence.
Source:
https://k.sina.com.cn/article_1673193460_63bae7f400100xr5t.html