In recent years, the treatment of patients with advanced cancer has blindly carried out too many operations, chemotherapy and radiotherapy, and blindly pursued the elimination of tumors in an attempt to achieve the goal of complete rehabilitation. However, as we all know, due to the poor body status of patients with advanced cancer, excessive treatment will seriously damage their resistance, but they are prone to repeated attacks and accelerate their death.
Experts from the cancer free home point out that the treatment of intermediate and advanced cancers should not blindly pursue the regression of tumors, but should focus on controlling tumors, prolonging the survival time of patients, realizing long-term "survival with tumors", striving for "peaceful coexistence" with tumor cells, and turning cancer into common chronic diseases such as diabetes and heart disease, which is the correct concept of scientific anti-cancer.
In the past decade, with the rise of immune drugs and targeted drugs, a large number of targeted drugs and immune drugs have been listed. In addition, medical researchers in various countries are also actively studying various black technologies for cancer treatment. Once regarded as a myth, refractory cancer is gradually changing towards chronic disease.
Proceeding from the national conditions, there is a big gap between China's medical treatment and that of developed countries. Many new drugs and new therapies are costly and expensive, and cancer friends can only sigh with admiration. But in the past two years, our country has accelerated the approval of new drugs and the research and development of new technologies. In the middle of 2022, a number of new drugs and technologies will be launched in China, and all of them happen to have clinical recruitment in China, especially the rapid development of cellular immunotherapy! So, the cancer free home editor can't wait to introduce these international advanced cutting-edge new technologies and new therapies to you today!
Cellular immunotherapy
Cellular immunotherapy uses the self-protection and killing ability of the human immune system to achieve the anti-tumor effect. The immune function cells in the patient are cultured and proliferated in vitro, and they have the ability to attack tumor cells through technical means, and then they are imported into the patient to achieve their own anti-cancer. This therapy is aimed at immune cells, not cancer cells, and does not cause great harm to the patient's body like surgery, radiotherapy and chemotherapy. It can be directly treated for early cancer patients, especially with the attack effect on residual cancer cells after surgery.
Judging from the rapid development of scientific research and clinical practice in recent years, cellular immunotherapy has high hopes in accuracy, effectiveness and safety, and is expected to rapidly rise to become the fourth pillar of anti-cancer therapy. In particular, by 2022, cellular immunotherapy will continue the strong momentum of 2021 and develop more rapidly. In addition, a variety of therapies are being recruited in China, covering patients with various types of cancer, so that more and more Chinese cancer patients can benefit from clinical treatment!
01. Car-t cell therapy
Car-t therapy is chimeric antigen receptor T cell immunotherapy, which is a new type of targeted therapy for tumor treatment. T cells are activated by genetic engineering technology and equipped with the positioning and navigation device car (tumor chimeric antigen receptor), which transforms the ordinary "soldier" T cells into "super soldiers", that is, car-t cells, which specifically recognize tumor cells in the body and efficiently kill tumor cells, so as to achieve the purpose of treating malignant tumors.
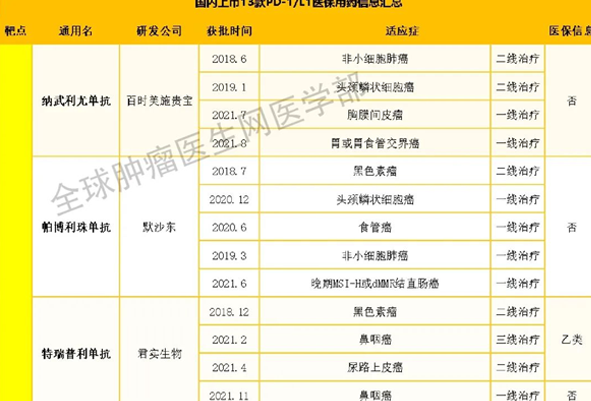
At present, with the approval for marketing of acesulfame injection and acesulfame injection in China in 2021, and the approval for marketing of cedacesulfame injection by FDA in 2022, car-t therapy has entered the blowout period. In particular, car-t technology has shown excellent efficacy in a variety of blood tumors, such as leukemia, lymphoma and multiple myeloma, which has enabled more than half of the blood cancer patients who are on the verge of extinction and have no drug to cure to obtain good efficacy.
In addition, car-t therapy has made remarkable progress in gastric cancer, liver cancer, pancreatic cancer and other fields. Scholars at home and abroad have made a variety of modifications to car-t, and constantly found new targets for the treatment of a variety of solid tumors.
01. burst! The first BCMA car-t therapy in China and the second in the world was approved by FDA
On February 28, 2022, the BCMA car-t product carvykti (trade name carvykti), which was jointly developed by Janssen and legendary biology, has been approved by the US FDA to be listed for the treatment of adult patients with recurrent / refractory multiple myeloma (mm).
This is the first cell therapy product approved by FDA in China and the second car-t cell immunotherapy approved to target BCMA in the world.

BCMA car-t therapy report
Cilta cel is an investigational B cell maturation antigen (BCMA) - directed car-t therapy for the treatment of recurrent or refractory multiple myeloma (RRMM).
The latest results reported at the ash conference in 2021 showed that after a median 22 month long-term follow-up, the objective response rate (ORR) reached 98%, and 83% of patients reached strict complete response (SCR), emphasizing that the remission deepened with time (SCR increased from 67% reported at the ash annual conference in 2020 to 83%). At 18 months, 66% of the patients survived without disease progression. The two-year progression free survival rate and overall survival rate were 61% and 74%, respectively. The latest research results to be released at the ASCO annual meeting in 2021 show that the median follow-up of 18 months has an overall survival (OS) rate of 81%, and the remission rate is comparable among all pre designated subgroups and patients with different treatment lines.
How to seek car-t cell therapy > >
Since the launch of the BCMA targeted sidakiolanza by FDA, the group of patients with relapsed or refractory multiple myeloma has received special attention. For the second most common malignant tumor in the blood system, car-t therapy undoubtedly provides cancer friends with new treatment options!
At present, cancer free home also has two car-t therapies for multiple myeloma and a three target car-t clinical trial targeting cd19/cd20/cd22. The car-t clinical trial for patients with B-cell acute lymphoblastic leukemia is under clinical recruitment. Patients who want to participate can submit pathological reports, treatment experience, discharge summary and other information to cancer free home medical department for preliminary assessment of their condition.
02. sword finger gastric cancer, domestic car-t therapy ct041 enters the confirmatory phase II clinical trial
As the first car-t cell targeting claudin18.2 in the world, ct041 came to the fore as early as the 2019 ASCO annual meeting. At that time, the total objective response rate was 33.3%, which had already amazed the world. Now, the more significant curative effect is undoubtedly the icing on the cake! This clinical data shows a good prospect for the treatment of digestive system tumors!
On may9,2022, the research results of car-t cell product ct041 of Keji pharmaceutical for the treatment of digestive system tumors were published in the international top journal Nature Medicine, which is the first clinical study of car-t cell treatment of solid tumors with the largest sample size published in the top academic journal so far!
The research data is particularly bright
1) The objective remission rate of all patients was 48.6%, and the disease control rate was 73%; The overall objective remission rate of all patients with gastric cancer was 57.1%, and the disease control rate was 75.0%.
2) The objective remission rate was 61.1%, and the disease control rate was 83.3%.
3) And the overall tolerance is good!
By March 3, 2022, ct041 has become the first and only car-t cell candidate product for the treatment of solid tumors that has entered the confirmatory phase II clinical trial in the world. This is a milestone for car-t to conquer the field of solid tumors. Congratulations!
In addition, there are many car-t cell therapies targeting cldn18.2. For example, lb-1904, developed by legendary biology, is used to treat gastric cancer or pancreatic cancer and has now entered phase I clinical trials. In addition, the first monoclonal antibody ab011 against claudin18.2 independently developed in China is used to treat patients with advanced gastric adenocarcinoma and solid tumor. At present, it is also being recruited for clinical trials.
At present, B-cell lymphoma, T-cell lymphoma, T-cell leukemia (T-ALL), acute leukemia, non Hodgkin's lymphoma, liver cancer, gastric cancer, prostate cancer, thyroid cancer and other cancers are urgently needed!
If you want to evaluate whether your condition can accept car-t therapy, you can submit the pathological report, treatment experience and discharge summary to the cancer free home medical department for preliminary evaluation!
03. targeting colorectal cancer! Stanza solid tumor car-t therapy Shine International
On May 17, 2022, Shanghai stanza biological company announced that it would attend the 25th annual meeting of American Society for gene and cell therapy (asgct) held in Washington from May 15 to May 19, 2022 and make an oral report.
At the meeting, stanza will develop coupledcar based on independent research and development ® Report on gcc19cart, the first candidate product developed by the platform technology, which is developed to treat patients with recurrent / refractory colorectal cancer (r/r mCRC).
On April 19, 2022, Shanghai stanza bio announced that its developed solid tumor car-t product gcc19cart was granted the fast track qualification by the U.S. Food and Drug Administration (FDA).
Gcc19cart is an autologous car-t treatment product, which is a leading solid tumor therapy for the treatment of recurrent, refractory and metastatic colorectal cancer (r/r mCRC). It has to be said that this honor has made car-t therapy take another solid step on the road of overcoming solid tumors. Congratulations!
According to the editor of cancer free home, at this asgct meeting, stanza will focus on introducing the data of 21 patients in 2 dose climbing test groups from 5 clinical centers in China, verifying the safety and preliminary efficacy of gcc19cart product. Among them, 13 patients were enrolled at level 1 dose (1x106 cells/kg) and 8 patients were enrolled at level 2 dose (2x106 cells/kg). According to the criteria for evaluating the efficacy of solid tumors (recist1.1), the objective response rate (ORR) in the level 1 dose group was 15.4% (2/13) and that in the level 2 dose group was 50% (4/8).
Clinical recruitment of cancer free home
At present, the car-t cell therapy of stanza is mainly about the clinical research of advanced solid tumors.
It covers 14 types of cancer, including colorectal cancer, lung cancer, breast cancer, liver cancer, bladder cancer cancer, esophageal cancer, kidney cancer, ovarian cancer, uterine cancer, melanoma, gastric cancer, pancreatic cancer, synovial sarcoma, head and neck cancer.
For specific inclusion criteria, please consult the cancer free home medical department for details, and a detailed medical evaluation will be conducted.
02. Tcr-t treatment
Car-t cells and tcr-t cells belong to T cells modified by genetic engineering technology. Compared with car-t therapy, tcr-t therapy has unique advantages in the field of solid tumor treatment.
Tcr-t cell therapy can recognize tumor specific antigens on the surface of cell membrane or from intracellular sources. It has entered clinical application from the initial basic immune research, showing preliminary efficacy in solid tumors. It has become the T cell immunotherapy that is most likely to make a breakthrough in the field of solid tumors!
What is tcr-t cell therapy
The main mechanism of tcr-t technology is to introduce new genes into ordinary T cells, so that the modified T cells can express TCR (T cell receptor) that can effectively recognize tumor cells, so as to guide T cells to kill tumor cells.
Clinical situation of tcr-t products approved for liver cancer
On may9,2022, the scg101 autologous T cell injection of Sinopharm was approved by the Singapore food and Drug Administration (HSA) for clinical trial. On March 10, the drug evaluation center (CDE) of China's State Drug Administration announced that scg101 autologous T cell injection successfully obtained the implied license for clinical trials to treat hepatitis B virus (HBV) - related hepatocellular carcinoma (HCC).
Scg101 clinical trial license
▲ picture source: CDE official website
On April 20, 2022, the innovative product taest1901 injection of Xiangxue life science has obtained the clinical trial license of China National Drug Administration (nmpa), and is intended to be used to treat advanced liver cancer or other advanced tumors with hla-a*02:01 tissue genotype and positive AFP expression.
Taest1901 injection clinical trial approved
▲ picture source: CDE official website
Cancer free home tcr-t therapy recruitment
So is there a chance to try tcr-t therapy? Xiaobian: this brings good news. At present, a tcr-t therapy is being developed to recruit liver cancer patients for clinical trials. Patients who want to participate can consult the cancer free home medical department for detailed inclusion criteria.
03. Car-nk treatment
In the past two years, in addition to car-t therapy, another new cancer cell therapy - natural killer (NK) cell therapy has gradually attracted attention. The researchers said that NK cells have more potential as a cellular anti-cancer therapy, and it may be safer, cheaper and faster.
The success of car-t cell therapy has stimulated people's enthusiasm for using car gene to modify NK cells to enhance their tumor killing ability.
Car-nk is to use genetic engineering to add a chimeric antibody to NK cells that can recognize tumor cells and activate NK cells to kill tumor cells at the same time. In 2020, car-nk immunotherapy was incorporated into one of the ten generations of remarkable progress in the biomedical field by the authoritative academic journal Nature Medicine.
Patients who want to seek car-nk cell therapy and other new treatment technologies at home and abroad can first submit their medical records to the cancer free home medical department for preliminary evaluation.
The achievements of car-nk therapy in solid tumors should not be underestimated
1. Innovative car-nk therapy ft536 approved by FDA for clinical trials
On January 10, 2022, fate therapeutics announced that the US FDA had approved the new drug clinical research (ind) application of ft536. Ft536 is a "ready to use" chimeric antigen receptor (car) natural killer (NK) cell therapy derived from induced pluripotent stem cells (IPSC) after multiple engineering modifications.

Car-nk therapy ft536 approved clinical trial
Ft536 expresses a class I major histocompatibility complex (MHC) associated protein A and B (mica/micb) specifically targeted α 3 domain.
Mica and MICB are stress proteins, which are highly expressed in many solid tumors. Cancer cells often pass through mica/b α 1 and α Proteolytic shedding of the 2 domain to escape recognition by immune cells. Previous studies have found that mica/b targeted α 3 domain antibody can specifically prevent mica/b from shedding and restore NK cell-mediated immunity.
The approval of ft536 for clinical application indicates that mica and MICB are becoming exciting cancer immunotherapy targets in a wide range of solid tumors, and ft536 also represents a new treatment strategy targeting these stress-induced ligands. The tumor types of the multicenter phase I clinical trial of this therapy include advanced non-small cell lung cancer, colorectal cancer, head and neck cancer, gastric cancer, breast cancer, ovarian cancer and pancreatic cancer.
2. "Spot" car-nk therapy launched an attack on "cancer king" pancreatic cancer
On January 6, 2022, the international authoritative journal of Gastroenterology published the heavy research jointly developed by the city of Hope National Medical Center and Cytoimmune therapeutics, a cell therapy innovation company. The research results showed that the "off the shelf" car-nk therapy targeting prostate stem cell antigen (PSCA) could significantly inhibit pancreatic cancer. This therapeutic method, known as cyto nk-203, is a ready-made allogeneic car-nk cell therapy, derived from umbilical cord blood, and a potential therapy to improve the safety and killing efficacy of NK cells.
Cyto nk-203 has a significant effect in the mouse model of human metastatic pancreatic cancer. It continues to survive in mice for more than 90 days, significantly prolongs their life span, and does not show treatment-related toxicity. This preclinical study supports the entry of this therapy into human clinical trials this year.
The research team believes that this car-nk therapy is promising for the treatment of pancreatic cancer for two reasons: first, the therapy is based on precision medicine and targets the special target of pancreatic cancer patients - PSCA; This is an immunotherapy that uses engineered human natural killer cells, which are engineered to specifically attack cancer cells.
In addition, because prostate stem cell antigen (PSCA) is also highly expressed in gastric cancer and prostate cancer, this car-nk cell therapy is also promising for the treatment of gastric cancer and prostate cancer.
In addition to the domestic clinical studies mentioned above, car-nk cells also have a good effect on ovarian cancer, colorectal cancer, non-small cell lung cancer, glioblastoma, neuroblastoma, liver cancer, etc., specifically recognize and efficiently kill breast cancer tumor cells, and have a significant effect on multiple myeloma.
Broad spectrum anticancer drugs
Recently, cancer targeted therapy has made rapid progress. Many highly effective targeted drugs have been approved to market or put into use at home and abroad. At the recent annual meeting of the American Association for cancer research (AACR) in 2022, many targeted drugs that have attracted the attention of cancer friends have sprung up, which has rekindled the hope of life for countless cancer friends. So, today's Xiaobian of cancer free home reviews the latest clinical studies of popular targeted drugs.
"Diamond" target ntrk
The first reason why ntrk is called "diamond" gene is that it is very rare. In the common lung cancer, breast cancer and colorectal cancer in China, only 1%~5% of patients have this mutation, while in some rare cancers, such as infantile fibrosarcoma and secretory breast cancer, the frequency of ntrk fusion is as high as 90%~100%. Secondly, the drugs listed and under development for ntrk fusion mutations have significant clinical effects. Patients receiving treatment can usually take effect quickly. Many late stage patients have been reborn after using ntrk inhibitors, which is as rare and precious as diamonds.
Heavyweight! Larotinib, a "legendary" anticancer drug sweeping 21 types of cancer, is listed in China
As the world's first targeted drug for initial treatment regardless of tumor source, larotinib was approved by FDA on november26,2018 for the treatment of solid tumors with neurotrophic receptor tyrosine kinase (ntrk) gene fusion in adults and children.
On April 13, 2022, the "legendary" anticancer drug larotrectinib (larotinib sulfate, trade name: vitrakvi), which Chinese cancer friends had been waiting for for for more than three years, was finally approved by the State Drug Administration of China (nmpa) to be listed for the treatment of adults and children with solid tumors carrying ntrk fusion gene. This shows that Chinese cancer friends have welcomed the world's first oral Trk inhibitor specially designed for cancer patients with ntrk gene fusion.
Up to now, ntrk fusion has been found in more than 25 types of cancer, including breast cancer, colorectal cancer, lung cancer, thyroid cancer, etc., which can be used by both adult and child patients.
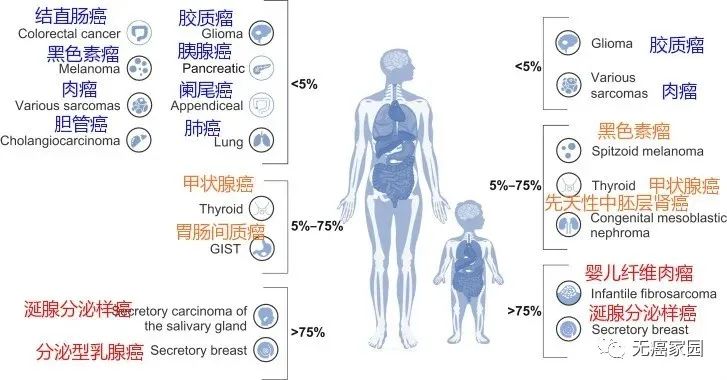
Probability of ntrk mutation in each cancer species
In addition to larotinib, the global research and development of ntrk drugs is in full swing, bringing new hope to more patients! Cancer free home has specially sorted out the information of ntrk inhibitors currently being recruited for cancer friends, hoping to give more treatment options to patients who are eager to seek medical advice!
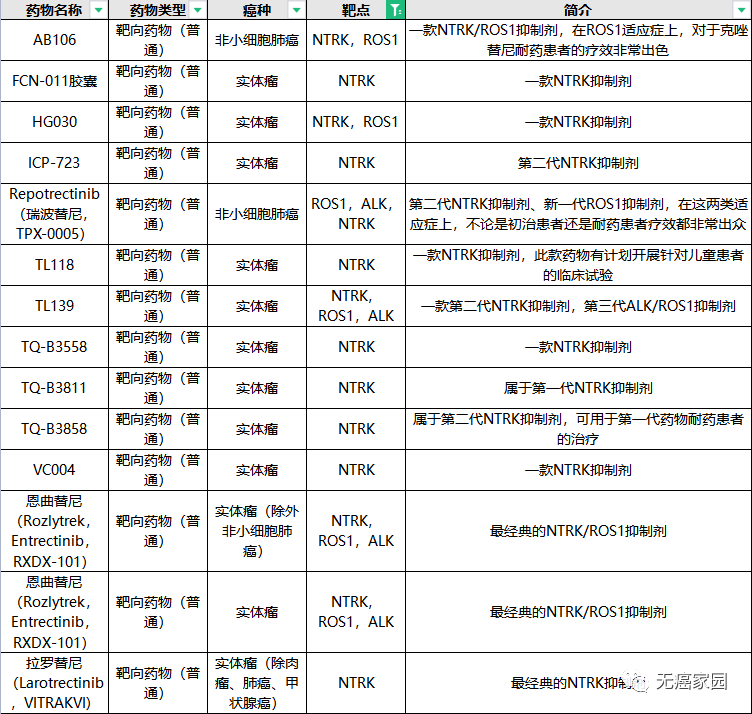
Ntrk clinical trial
Target of "nail households" - KRAS
KRAS is one of the most common oncogenes in solid tumors. About 30% of tumors have KRAS mutations, including 90% of pancreatic cancer, 30%~40% of colon cancer and 15%~20% of lung cancer. However, there are few KRAS targeted drugs, and KRAS once became the most difficult mutation without drugs.
The disease control rate was 85.7%, and the domestic targeted drug d-1553 made its debut in AACR
D-1553 is a new, efficient and oral KRAS G12C inhibitor independently developed by Yifang biology. Recently, at the annual meeting of the American Association for cancer research (AACR) in 2022, Yifang biological first released the clinical phase I data of its oral KRAS G12C inhibitor d-1553 in cancer patients, making d-1553 the first domestic KRAS inhibitor to publish clinical data.
In an international multicenter phase I study of patients with advanced or metastatic solid tumors carrying KRAS G12C mutations, d-1553 was well tolerated in 22 patients without any dose limiting toxicity. In 21 evaluable patients, 19.0% of the confirmed objective tumor remission rate was observed, reaching 85.7% of the disease control rate. Tumor remission was observed at dose levels as low as 300mg per day.
In another study conducted by Professor Lushun of Shanghai Chest Hospital as the main researcher, 59 patients with non-small cell lung cancer (NSCLC) carrying KRAS G12C mutation were included in the analysis, including 52 assessable patients. The objective tumor remission rate reached 40.4% and the disease control rate reached 90.4%. These patients are all patients with advanced or metastatic cancer, and most of them have received second-line or more systemic anti-cancer drugs.
The data showed that under the pr2d (600mg/bid, bid twice a day) dose, the objective remission rate of d-1553 in 32 patients was 40.6% and the disease control rate was 84.4%. In terms of safety, d-1553 was well tolerated and did not reach the dose limiting toxicity.
It is reported that d-1553 has been approved by FDA in October, 2020. It has launched international multicenter phase i/ii clinical trials in the United States, Australia, Taiwan, China, South Korea and other countries and regions. At present, it is progressing smoothly. In january2021, it was approved by the drug evaluation center of the State Drug Administration of China to carry out phase i/ii clinical trials.
The good news is that d-1553 has officially started recruiting patients for all kinds of solid tumor patients with KRAS G12C mutation!
If you are one of the following types of cancer, please contact us immediately
At present, KRAS G12C patients with colon cancer, pancreatic cancer, cholangiocarcinoma, endometrial cancer and ovarian cancer are in urgent need. If you want to participate, you can consult the cancer free home medical department to understand the trial.
In addition to lumakras and d-1553, with the continuous efforts of medical researchers, the fortress KRAS was finally broken, and a number of new drugs were emerging. Several drugs under research have achieved early success in the clinical research stage, including adagrasib (mrtx849), jnj-74699157 (ars-3248), jab-3312, ly3499446 and pan KRAS inhibitor Bi 1701963.
Diamond mutation - ALK
It is lucky for the patients who are identified as ALK positive, because the targeted drugs for ALK are highly effective and have little side effects. If they do not pay attention to the tumor, they will be "eaten away". The fusion mutation of ALK gene in lung cancer exists in about 3%~7% of non-small cell lung cancer, and the number of patients is not large. However, in patients with advanced non-small cell lung cancer with ALK mutation, the 5-year survival rate was more than 60% with ALK inhibitor aletinib or kezotinib. Therefore, ALK mutation is also called "Diamond mutation" because of its rare but effective characteristics.
The positive rate of ALK fusion gene in Chinese lung adenocarcinoma was 5.1%, while the positive rate of ALK fusion gene in Chinese adenocarcinoma patients with wild-type EGFR and KRAS was as high as 30%~42%. For non-small cell lung cancer, young, female, Asian non-smoking patients are more likely to have ALK gene mutations.
Finally! Laratinib, the third generation ALK inhibitor, was approved to be listed in China
On April 28, 2022, Pfizer's ALK inhibitor loratinib was approved by the State Drug Administration (nmpa) to be listed for the treatment of ALK positive advanced non-small cell lung cancer. This is also the first approved third-generation ALK inhibitor in China.
According to the clinical trial data of loratinib in the treatment of Chinese patients, the overall remission rate was 70.1%, of which the complete remission rate was 11.9%; In patients with brain metastases at baseline, the overall remission rate of intracranial lesions was 80.6%, and the complete remission rate was as high as 52.8%.
The overall remission rate of patients treated with loratinib was 47.6%, of which the complete remission rate was 4.8%; In patients with brain metastases at baseline, the overall remission rate of intracranial lesions was 47.6%, of which the complete remission rate was 28.6%.
At present, cancer free home has a number of targeted drugs for ALK mutation (only part of the show) under Recruitment!
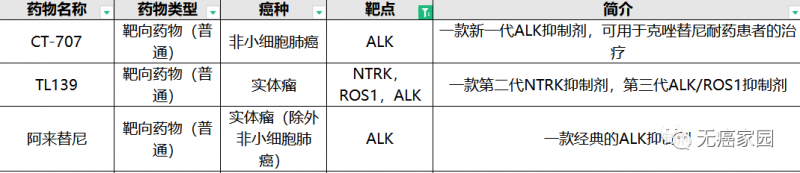
ALK clinical trial
If you want to evaluate whether your condition meets the above recruitment conditions, you can submit the pathological report, treatment experience and discharge summary to the cancer free home medical department for preliminary evaluation!
Hot target - FGFR
FGFR gene mutations are commonly found in solid tumors such as lung cancer, liver cancer, intrahepatic cholangiocarcinoma, breast cancer, gastric cancer, uterine cancer and urothelial carcinoma, and there are differences in FGFR mutation types and frequencies among different cancer species.
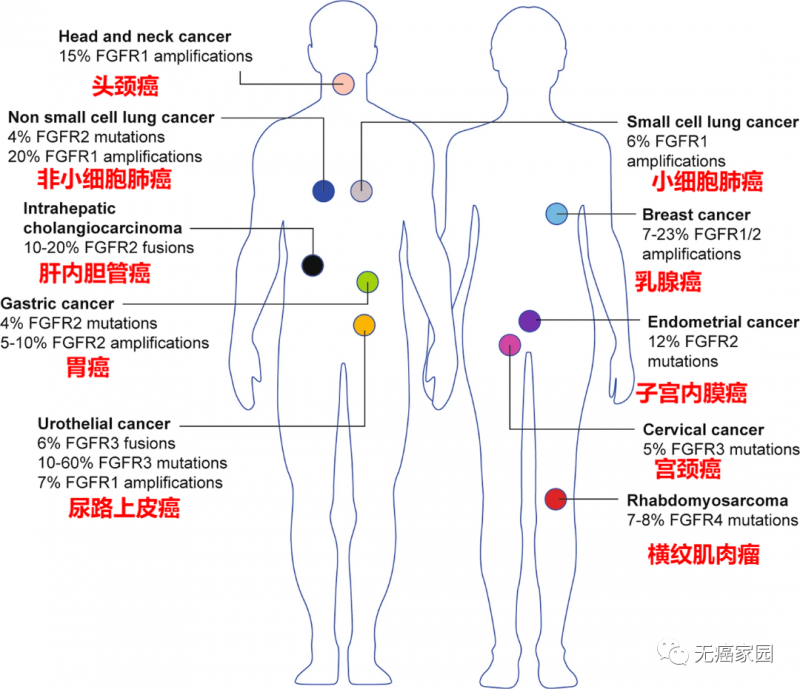
Types of cancer with FGFR changes
The disease control rate is 100%! Pemigatinib, the first targeted drug in the history of cholangiocarcinoma in China, made a brilliant debut
On April 6, 2022, Cinda bio announced that its imported pemigatinib (trade name: dabetan) was approved to be listed in China for the treatment of adult patients with advanced, metastatic or unresectable cholangiocarcinoma who had received at least one systematic treatment in the past and were confirmed to have FGFR2 fusion or rearrangement.

Pemigatinib approved clinical trial

Approval of pemigatinib clinical trial
Screenshot from nmpa official website
This exciting news means that the first FGFR inhibitor approved for listing in China has finally come. Its listing fills the blank of targeted therapy for cholangiocarcinoma in China and also means the end of the era of chemotherapy only.
At the same time, FGFR targeted drugs have gradually entered the public's field of vision. You cancer friends may be unfamiliar with FGFR targets. However, as one of the hot targets of "unlimited cancer" therapy, the indications of FGFR (fibroblast growth factor receptor) on the market mainly focus on cholangiocarcinoma and urothelial carcinoma. In addition, the target covers more than 16 kinds of cancer, mainly including lung squamous cell carcinoma, liver cancer, gastric cancer, breast cancer and other solid tumors.
In order to facilitate cancer friends to have a more intuitive understanding of the clinical trials of FGFR inhibitors, Xiaobian specially sorted out the clinical trials currently being recruited by cancer free home, which covered solid tumor types such as cholangiocarcinoma, liver cancer and gastric cancer.
If you want to evaluate whether your condition can accept the clinical trial of new drugs, you can submit the pathological report, treatment experience and discharge summary to the cancer free home medical department for preliminary evaluation!
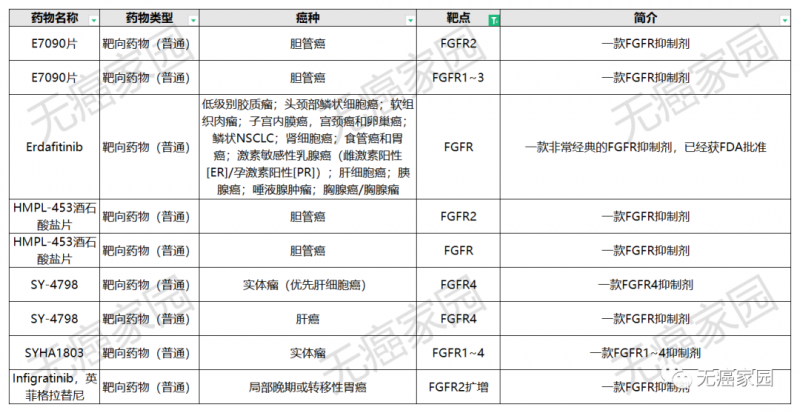
FGFR inhibitor clinical trial
Xiaobian has something to say
In recent years, due to the development of medical treatment, new cancer treatment methods and new anticancer drugs have emerged one after another. The research and development of cellular immunotherapy and targeted drugs are in full swing, bringing new hope to cancer patients.
According to the latest statistical data, compared with patients with advanced lung cancer, gastric cancer and liver cancer who only receive surgery, radiotherapy and chemotherapy, adjuvant cellular immunotherapy or targeted therapy can prolong the survival time of patients and significantly improve the data effect.
In fact, tumor treatment is actually a complex "project". It is difficult to achieve good results simply by relying on a certain treatment method. Tumor treatment requires the cooperation of various treatment methods to achieve the final victory in the fight against cancer.
Article source:
http://www.globecancer.com/azzx/show.php?itemid=15257