Recently, the 2022 annual meeting of the American Cancer Research Association came to a successful conclusion. AACR (American Research Association) is the earliest and largest scientific organization dedicated to comprehensive, innovative and high-level cancer research in the world. The latest progress in cancer treatment will be announced at the AACR annual meeting every year. This international event in 2022 can be described as a feast. Many new drugs and new therapies have appeared one after another to declare war on cancer.
The medical department of the global oncologist network reviewed the eight most noteworthy developments at the meeting, covering common types of cancer, hoping to bring you confidence in overcoming cancer.
AACR conference 2022
New drugs for lung cancer
01. Breaking the dilemma of ositinib resistance and the dawn of the fourth generation EGFR targeted drugs
Blu-945 is the fourth generation EGFR TKI developed by the famous blueprint company in the United States. At the AACR conference in 2022, the symphony research results first carried out by blu-945 once again brought surprises, showing that it also showed encouraging efficacy in the population resistant to ositinib.
As of march9,2022, 33 patients with EGFR mutant NSCLC received five different doses (range: 25-400 mg QD) of blu-945. It is worth mentioning that most patients (79%) have received at least three-line system treatment before, and up to 97% of patients have received ositinib, which can be said to be advanced patients without better targeted treatment options in clinic.
The results show that:
1. good safety tolerance
Blu-945 is generally well tolerated at all test doses.
2. strong anti-tumor activity and obvious tumor shrinkage
Among the patients who had undergone a large number of pretreatment, the higher dose of blu-945 had stronger antitumor activity. Tumor shrinkage was observed in patients treated with 200-400 mg QD. (unconfirmed PR 1 occurred in one patient who received 400 mg QD. This patient carried exon 19 deletion, T790M and c797s mutations, was resistant to platinum chemotherapy, erlotinib and ositinib, and responded well to blu-945).
3. the contents of tumor mutations T790M and c797s decreased significantly
Symphony test is also one of the first oncology studies to evaluate tumor biology and early drug activity by analyzing ctDNA (tumor circulating DNA) in plasma through real-time next-generation sequencing. CtDNA detection results can provide panoramic data of tumor in patients, and play an important role in tumor screening, prognosis and treatment. All patients were tested for ctDNA at baseline and each treatment cycle.
To the researchers' surprise, the ctDNA test results 14 days after blu-945 treatment showed that 83% (10/12) of the patients' peripheral blood samples had a decrease in T790M mutation abundance;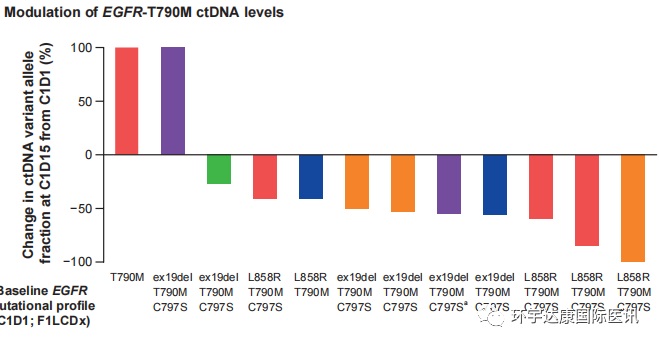
Blu-945 treatment data
The abundance of c797s mutations decreased in 81% (9/11) of patients. In all patients receiving 400mg QD, T790M and c797s ctDNA decreased, including 3 patients who met the clearance criteria (undetectable).
Blu-945 is a powerful and selective fourth generation EGFR-TKI developed by blueprint, which can target mutations carrying EGFR T790M and c797s. Compared with other fourth generation EGFR-TKIs under development, blu-945 is one of the four generation EGFR-TKIs with the fastest progress at present. Its preclinical data to overcome drug resistance show high antitumor activity. We also expect blu-945 to publish more positive clinical data as soon as possible and be approved for listing as soon as possible, bringing salvation to patients resistant to oshitinib.
02. Objective remission rate: 60.9%! Met+ patients with lung cancer will usher in a new therapy with lasting curative effect
Gumetinib (scc244) is a highly selective met inhibitor developed by the state. It has shown long-lasting efficacy in patients with non-small cell lung cancer carrying a jump mutation in met exon 14. The latest data of phase 1/2 code named glory test (nct04270591) released at the 2022 AACR conference will be transmitted to the good news.
A total of 163 patients were screened in this experiment, of which 73 non-small cell lung cancer with jump mutation in met exon 14 participated in the experiment and received gometinib. Almost all patients were from China (90.4%), and a small number were from Japan (9.6%). It is worth mentioning that 87.7% of the patients were locally advanced non-small cell lung cancer, and 13.7% had brain metastasis.
The results showed that the overall remission rate (ORR) was as high as 60.9% and the overall disease control rate was 82.6%! It is highly effective in both primary and treated populations.
Professor Lushun of Shanghai Chest Hospital said that "glory research has proved the high efficiency and durability of scc244, and also observed the preliminary effect on brain metastasis".
03. Double the survival rate! Strong inhibitor sotorasib rewrites the fate of KRAS lung cancer patients
At the AACR (American Association for cancer research) annual meeting in 2022, sotorasib (amg-510), the first "revolutionary anticancer drug" that is effective against KRAS mutation, released updated data. The results showed that sotorasib may provide long-term clinical benefits for patients with non-small cell lung cancer and refresh the survival hope of patients with advanced KRAS lung cancer.
Dr. grace K. dy of Roswell Park Cancer Institute said at the meeting, "long term follow-up data are very important for better determining the safety and efficacy of sotorasib, because it is a first-class KRAS G12C inhibitor therapy approved for this patient population!"
According to the data update of phase 1/2 codebreak 100 trial (nct03600883), a total of 174 kras+ non-small cell lung cancer patients were enrolled. It is worth mentioning that most of these patients have received an average of two treatment regimens, including pd-1/l1 and chemotherapy.
The results show that:
40.7% of the patients had partial or complete response to sotorasib, the disease control rate (DCR) was 84%, and the median response duration was 12.3 months. It is worth noting that the two-year overall survival rate is close to 33%, which is very favorable compared with historical control treatment. For example, the two-year overall survival rate of non squamous NSCLC patients treated with chemotherapy drug docetaxel with or without anti ramucirumab as second-line treatment is expected to be only 15% to 22%.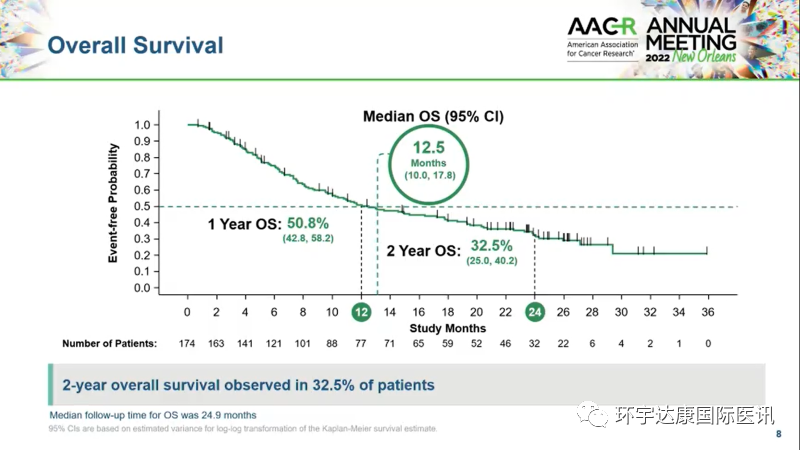
Sotorasib treatment data
New drugs for brain tumor
01. 90% of patients have clinical benefits! New car-t therapy challenges fatal brain tumors
At the AACR conference in 2022, the new car-t therapy targeting gD2 (gd2-car T) shocked four people! The researchers reported that gd2-car t therapy could treat diffuse midline gliomas with h3k27m mutations.
The results showed that among the 10 patients who received intravenous gD2 targeted car-t therapy, 9 patients obtained radiological and clinical benefits, and then they received subsequent intraventricular injection (ICV) of gD2 targeted car-t therapy. This shows that 90% of patients can benefit clinically. It is worth mentioning that the tumor volume of a 31 year old patient with diffuse midline glioma (dmg) decreased by more than 95% and almost disappeared; A 17-year-old patient with diffuse pontine gliomas (DIPG) had a pontine tumor volume reduction of more than 98% and almost disappeared.
At present, car-t therapy in China is mainly aimed at relapsed and refractory patients whose standard treatment fails (surgery, radiotherapy, chemotherapy, targeted therapy, PD-1 treatment, etc.), and there are few relevant car-t studies on glioma. Therefore, the international clinical research progress undoubtedly points out a way for us, and also gives glioma patients an additional treatment option.
New drugs for solid tumor
01. Disease control rate: 86%! A new generation of car-t therapy launches a fierce attack on solid tumors
Bnt211, a new generation car-t therapy, attracted much attention at this year's AACR Conference! This new therapy is composed of car T cell therapy targeting claudin-6 (CLDN6) and carvac, an mRNA vaccine encoding claudin-6 (CLDN6). The vaccine will express CLDN6 on the surface of antigen-presenting cells and stimulate the expansion of car-t cells in vivo.
The AACR Conference on April 10, 2022 announced the excellent data of the phase 1 clinical trial of bnt211 therapy (nct04503278).
By March 10, 2022, 16 patients with advanced solid tumors had been enrolled, including 8 patients with testicular cancer, 4 patients with ovarian cancer and 4 patients with other solid tumors (fallopian tube cancer, gastric cancer, sarcoma and endometrial cancer).
The results are very exciting! Among the 14 assessable patients, 6 patients had a reduction of at least 30% in tumor size and achieved partial remission (PR). One of the target lesions disappeared and achieved complete remission. 5 patients had stable disease (SD). The overall response rate (ORR) was 43% and the disease control rate was 86%.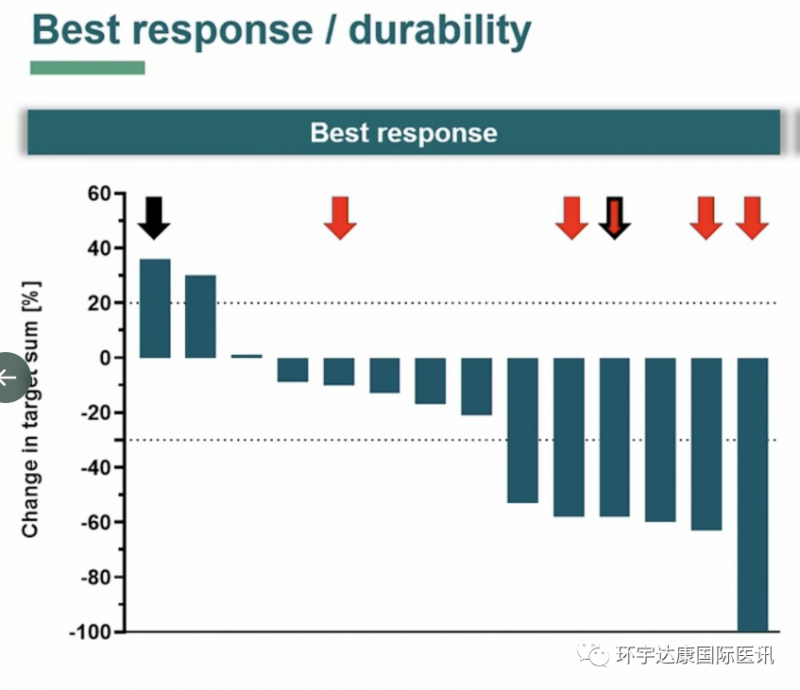
Bnt211 therapeutic data
02. The disease control rate was 85.7%. Domestic KRAS targeted drug d-1553 challenged solid tumors
D-1553 is a new, highly effective oral KRAS G12C inhibitor independently developed by Yifang biology. Recently, at the annual meeting of the American Association for cancer research (AACR) in 2022, Yifang biological first released the clinical phase I data of its oral KRAS G12C inhibitor d-1553 in patients with advanced solid tumors. The results showed that in 21 evaluable patients, 19.0% of the confirmed tumor objective remission rate was observed, reaching 85.7% of the disease control rate. Tumor remission was observed at dose levels as low as 300mg per day.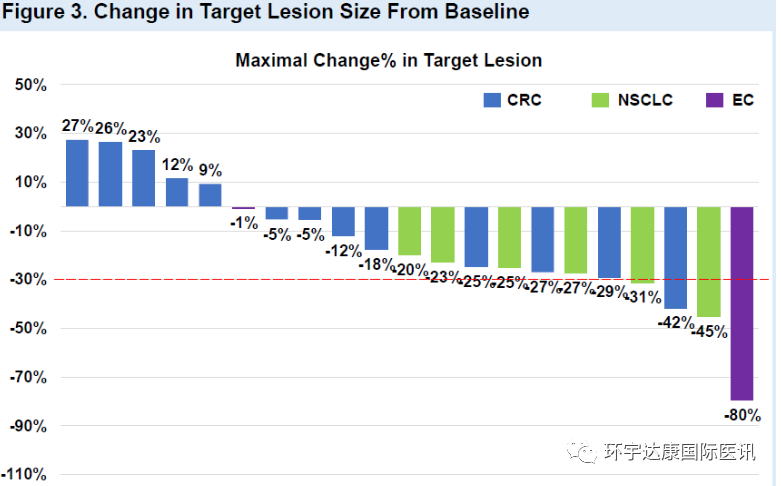
D-1553 treatment data
In another study on patients with non-small cell lung cancer (NSCLC) carrying KRAS G12C mutation, 59 patients were included, of which 52 were evaluable patients. The objective tumor remission rate reached 40.4% and the disease control rate reached 90.4%. These patients are all patients with advanced or metastatic cancer, and most of them have received second-line or more systemic anti-cancer drugs.
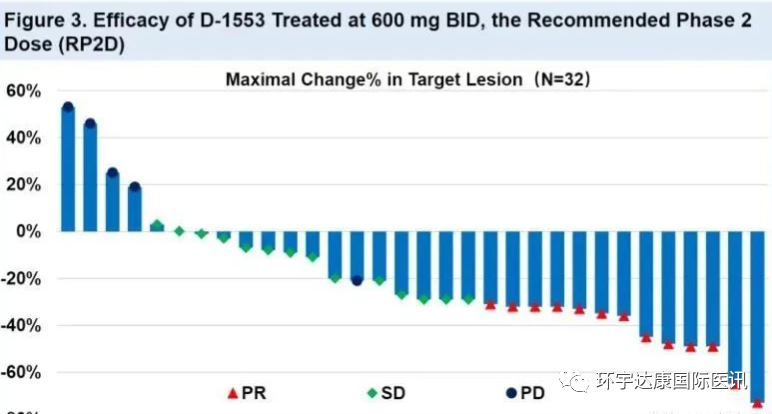
Data on the treatment of non-small cell lung cancer with d-1553
What is more exciting is that d-1553 has officially launched clinical research in China, targeting various solid tumor patients with KRAS G12C mutation. For example, colon cancer, pancreatic cancer, cholangiocarcinoma, endometrial cancer and ovarian cancer all have a high mutation rate of KRAS G12C. A large number of patients have successfully applied for enrollment through the medical department of the global oncologist network.
03. The second generation of domestic pan cancer targeted drug icp-723 appeared in AACR
At the AACR Conference on April 11, 2022, nuocheng Jianhua released the preclinical data of icp-723. The results showed that icp-723 could effectively inhibit the kinase activities of TrkA, TrkB and trkC. It has shown strong in vitro efficacy in trk driven tumors. Colleagues have also overcome the resistance mutations that often occur after the treatment of the first generation Trk inhibitors, such as TrkA g595r and trkC g623r/e.
Icp-723 is a new generation ntrk inhibitor independently developed in China. It can treat advanced or metastatic solid tumors carrying ntrk fusion gene, including breast cancer, colorectal cancer, lung cancer, thyroid cancer, and patients resistant to the first generation ntrk inhibitors larotinib and ntrotinib.
It is encouraging that, at present, icp-723 is in phase I dose increasing (1mg, 2mg, 3mg and 4mg) clinical trial in China, and according to the official press release, icp-723 has shown efficacy in two patients who are qualified for ntrk fusion. Ntrk fusion positive patients in the 3 mg cohort achieved stable disease (> 20% tumor reduction), and patients in the 4 mg cohort achieved partial remission at the end of cycle 1 or at the first tumor assessment on day 28. The good news is that this clinical trial is currently recruiting patients.
(Note: patients who have undergone genetic testing can take out the report to see if there is ntrk1/2/3 fusion, they can immediately contact the medical department of global oncologist network to apply for new drug treatment. Patients who do not understand the report can also call the medical department of global oncologist network to read the report.)
New drugs for hematological tumor
01. Objective remission rate 100%! New NK cell therapy will bring new hope to cancer patients
Patients receiving the recommended dose of phase 2 not only achieved 100% objective remission rate, but also 62% complete remission rate after two rounds of treatment!
At the AACR conference in 2022, the world-renowned American MD Anderson Cancer Center announced a project of natural killer (cbnk) cells from umbilical cord blood combined with congenital cell conjugates (ice ®) The preliminary clinical trial results of afm13 (cd16a / CD30) in the treatment of lymphoma: the objective remission rate reached 100%, which caused a great sensation.
A total of 22 patients with relapsed or refractory cd30+ Hodgkin's lymphoma and non Hodgkin's lymphoma were recruited in this study. It is worth mentioning that these patients had previously received an average of 7 pre-treatment regimens and developed resistance to therapies including antibody coupled drugs and PD-1 inhibitors. They can be said to be clinically incurable and refractory patients. Finally, 19 patients could be evaluated, of which 13 received the phase 2 recommended dose.
The results show that:
The overall response rate (ORR) of 19 patients was 89%, including 10 complete responses (CR). After receiving two rounds of treatment, all 13 patients who received the recommended dose of phase 2 responded, achieving 100% objective response rate (ORR), and the complete response rate increased from 38% to 62% after the first round of treatment.
The future has come, and cancer patients will usher in a good era when drugs are available
As these studies have shown, a variety of targeted and immunotherapies and combination therapies for a variety of cancer types and patient populations around the world are under intense research, aiming to bring better treatment options with fewer side effects to cancer patients.
What we need to tell you is that in the past, these new anti-cancer drugs developed and marketed in the United States were far away from domestic patients. In the past two years, with the attention of the state, the approval speed of R & D and marketing of various anti-cancer drugs has been accelerated, so that more foreign new and good drugs can also benefit domestic cancer patients. At the same time, the above-mentioned drugs have also carried out clinical trials in China, so that domestic patients can enjoy the cutting-edge treatment synchronized with the United States for the first time.
Patients who want to apply can contact the global oncologist network to conduct a preliminary evaluation by the medical department, and we will provide you with recruitment information in a timely manner.
We will wait and see, and look forward to the early launch of these new drugs for the benefit of the public!
Reference material:
https://www.aacrmeetingnews.org/news/positive-results-reported-from-four-clinical-trials-of-cellular-immunotherapies/
Article source:
http://www.globecancer.com/azzx/show.php?itemid=15100