The first FGFR inhibitor in China, pemigatinib and pemitinib, was approved for listing, and a number of FGFR gene mutation targeted drugs swept 16 major cancer species
Dabetan approved for listing
In recent years, with the double development of new drug research and development and gene detection technology, more and more "targets" have been discovered by medical researchers and developed effective drugs for clinical application, bringing hope for long-term survival to many imprisoned cancer patients.
On April 6, 2022, Cinda bio announced that its imported pemigatinib (trade name: dabetan) was approved to be listed in China for the treatment of adult patients with advanced, metastatic or unresectable cholangiocarcinoma who had received at least one systematic treatment in the past and were confirmed to have FGFR2 fusion or rearrangement.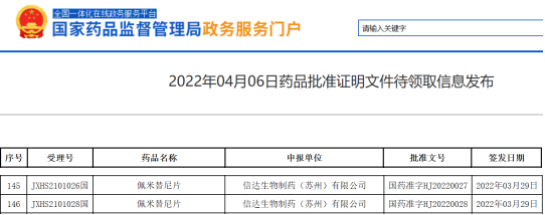
Pemetinib approved for listing
The screenshot comes from the nmpa official website. This exciting news means that the first FGFR inhibitor approved for listing in China has finally come. Its listing fills the gap in the targeted treatment of cholangiocarcinoma in China and also means the end of the era of chemotherapy only.
At the same time, FGFR targeted drugs have gradually entered the public's field of vision. You cancer friends may be unfamiliar with FGFR targets. However, as one of the hot targets of "unlimited cancer" therapy, the indications of FGFR (fibroblast growth factor receptor) on the market mainly focus on cholangiocarcinoma and urothelial carcinoma. In addition, the target covers more than 16 kinds of cancer, mainly including lung squamous cell carcinoma, liver cancer, gastric cancer, breast cancer and other solid tumors.
Emerging anticancer target: FGFR, do you know
FGFR gene family includes four subtypes FGFR1, FGFR2, FGFR3 and FGFR4 and some isomers. Common mutation types include gene amplification / fusion / deletion mutations. See the table below for details.
Common FGFR gene aberrations in tumors
FGFR gene mutations are commonly found in solid tumors such as lung cancer, liver cancer, intrahepatic cholangiocarcinoma, breast cancer, gastric cancer, uterine cancer and urothelial carcinoma, and there are differences in FGFR mutation types and frequencies among different cancer species.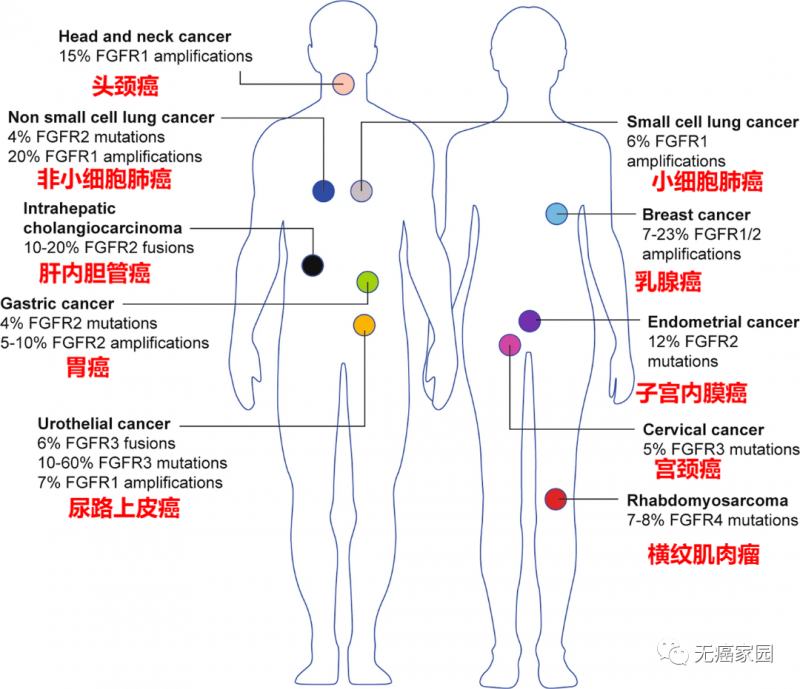
Types of cancer with FGFR changes
In a test result of next generation sequencing (NGS) of 4853 cancer species published in 2016, it was found that 360 FGFR abnormalities could be observed, with a frequency of 7.1%, especially in urothelial carcinoma (32%), breast cancer (18%), endometrial carcinoma (13%), squamous cell lung cancer (13%), ovarian carcinoma (9%), and cancer with unknown primary focus (8%).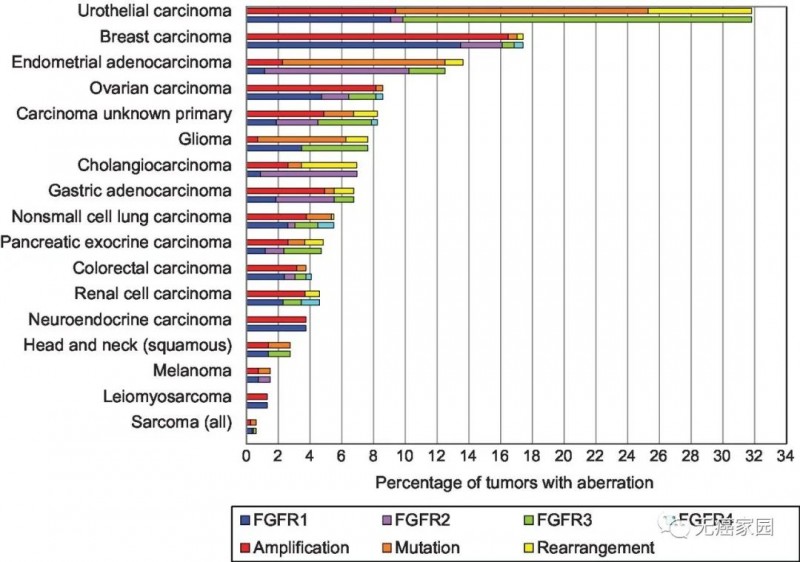
FGFR mutation probability
Among them, 66% were gene amplification, 26% were gene mutation, and 8% were chromosome rearrangement. The variation rate of FGFR1 is much higher than that of the other three subtypes, accounting for 49%, that of FGFR2 is 19%, that of FGFR3 and FGFR4 are 26% and 7% respectively, and about 5% of patients have more than one FGFR subtype variation.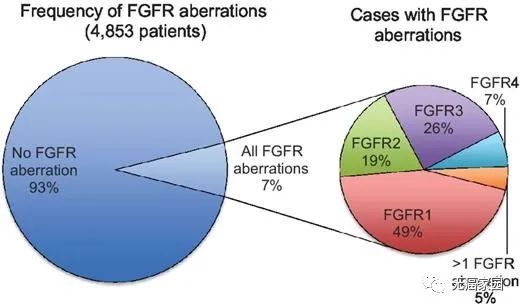
FGFR variation rate
In a genome analysis study involving 16 types of cancer and targeting 10582 Chinese cancer patients, FGFR abnormalities were found in 745 patients, equivalent to a prevalence of 7.0%. Most of the aberrations occurred in FGFR1 (56.8%), followed by FGFR3 (17.7%), FGFR2 (14.4%) and FGFR4 (2.8%). About 8.5% of the patients had two or more variants. For the Chinese population, the relatively small number of cancers with FGFR mutations include colorectal cancer (31%), gastric cancer (16.8%), breast cancer (14.3%) and esophageal cancer (12.7%).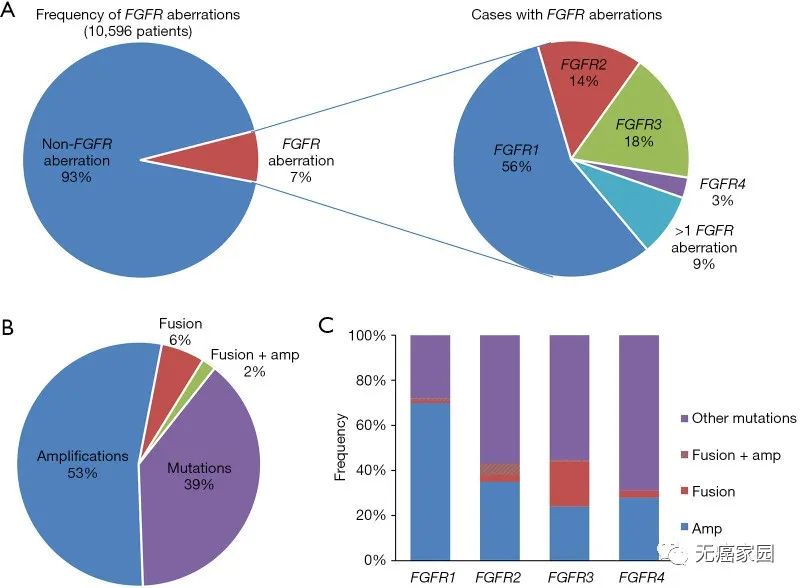
Prevalence of FGFR
Common cancer species with fgfr1~4 gene aberrations
●FGFR1
△ FGFR1 gene amplification has been confirmed to be found in different tumor types, including squamous non-small cell (17%) and small cell lung cancer (6%), estrogen receptor positive breast cancer (10%), ovarian cancer (5%) and bladder cancer (<2%).
△ it is reported that FGFR1 gene fusion has been found in lung squamous cell carcinoma, breast cancer, glioblastoma and brain tumors.
●FGFR2
△ FGFR2 gene amplification has been reported in triple negative breast cancer (4%) and poor prognosis gastric cancer (7%~9%). FGFR2 gene mutations were found in endometrial carcinoma (12%), lung cancer (4%~5%), gastric cancer (10%) and urothelial carcinoma (<2%).
△ FGFR2 fusion protein exists in lung adenocarcinoma, squamous cell carcinoma, thyroid carcinoma, prostate carcinoma and cholangiocarcinoma.
●FGFR3
△ FGFR3 amplification was found in urothelial carcinoma, breast cancer, ovarian carcinoma, gallbladder carcinoma and adenoid cystic carcinoma.
△ FGFR3 gene mutations exist in 70% of non muscle invasive tumors and 20% of invasive bladder tumors, cervical cancer, multiple myeloma and oral squamous cell carcinoma.
●FGFR4
△ FGFR4 kinase domain activating mutations were found in rhabdomyosarcoma (7%~8%), malignant lung adenocarcinoma, malignant glioma, breast cancer and other tumors.
△ abnormal overexpression of FGFR4 was found in 39% of patients with esophageal squamous cell carcinoma and 82% of patients with malignant peripheral schwannoma. In addition, the overexpression of FGFR4 was also found in ovarian cancer, gastric cancer, pancreatic cancer, colon cancer, etc.
In order to facilitate cancer friends to have a more intuitive understanding of the clinical trials of FGFR inhibitors, Xiaobian specially sorted out the clinical trials currently being recruited by cancer free home, which covered solid tumor types such as cholangiocarcinoma, liver cancer and gastric cancer.
If you want to evaluate whether your condition can accept the clinical trial of new drugs, you can submit the pathological report, treatment experience and discharge summary to the cancer free home medical department for preliminary evaluation!
FGFR clinical trial
A number of popular FGFR inhibitors on the market and under development appeared in turn
Next, the editor of cancer free home will share with you a number of popular FGFR inhibitor drugs listed and under development for your reference.
Three marketed FGFR targeted drugs
Up to now, there are three approved pan FGFR inhibitors in the world: pemazyre (i.e. pemigatinib) of Incyte, balversa (erdatinib) of Johnson & Johnson and infigratinib (infigratinib) of QED therapeutics.
The first cholangiocarcinoma targeted drug: pemigatinib
Some studies have shown that about 40% of patients with cholangiocarcinoma have potential targeted gene mutations, which has the possibility of targeted therapy. The main Mutation Genes in patients with cholangiocarcinoma include KRAS, BRAF, FGFR2, idh1/2, etc., of which FGFR2 mutation is the most important.
On April 6, 2022, pemigatinib, the FGFR 1/2/3 inhibitor of Cinda bio, was approved by the State Drug Administration for listing, becoming the first FGFR inhibitor approved for listing in China.
Pemigatinib
The results of the phase 2 study of pemigatinib in Chinese patients with advanced cholangiocarcinoma published in September, 2021 show that:
Among the 30 assessable patients, 15 patients achieved disease remission, with an objective remission rate (ORR) of 50% and a disease control rate (DCR) of 100%. In addition, pemigatinib also shows good security.
In fact, as early as April 17, 2020, FDA accelerated the approval of pemazyre (pemigatinib) of Incyte company for the treatment of previously treated patients with locally advanced or metastatic cholangiocarcinoma carrying fibroblast growth factor receptor 2 (FGFR2) fusion or rearrangement. The approval time was one and a half months ahead of the scheduled approval date of May 30, which was also the first targeted therapy for cholangiocarcinoma approved by FDA.
Pemigatinib is a powerful selective oral inhibitor targeting FGFR subtype 1/2/3. It has been confirmed in previous clinical studies that the drug has selective pharmacological activity against FGFR gene mutated tumor cells. The second-line treatment of cholangiocarcinoma with an orr of 35.5% and a DCR of 82% has become the first targeted drug in the history of cholangiocarcinoma! It has epoch-making significance!
A total of 146 patients with advanced cholangiocarcinoma who had received ≥ 1-line treatment were included in the light-202 trial and divided into 3 cohorts: a was FGFR2 fusion / rearrangement (107 cases), B was other FGFR mutations (20 cases), C was non FGFR mutations (18 cases), and 1 patient was undetermined. All patients received pemigatinib (13.5mg, once a day, 2 weeks of use and 1 week of rest).
The results showed that the objective response rate (ORR) in group A was 35.5%, of which 3 patients (2.8%) had complete response (CR), 35 patients (32.7%) had partial response (PR), and 50 patients (46.7%) had stable disease. The disease control rate was 82%. The orr for groups B and C was 0%. Compared with the other two cohorts, the proportion of patients in cohort a who responded effectively to drugs was much larger and the effective time was much longer.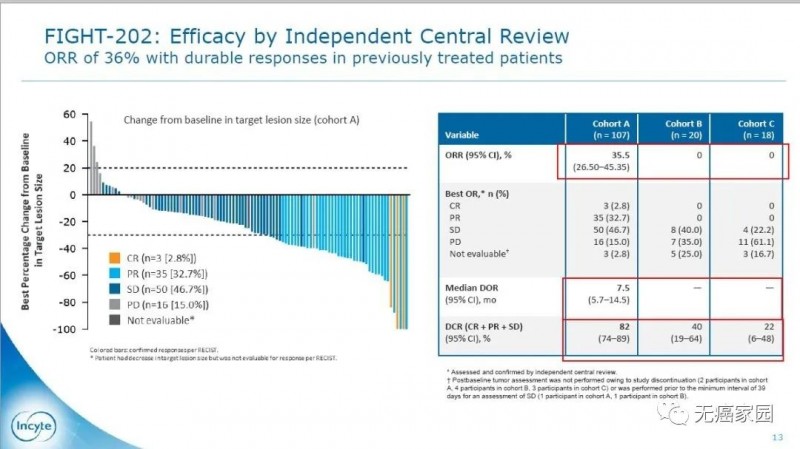
Pemigatinib response rate
The median dor of group A was 7.5 months, and the median PFS and OS were 6.9 and 21.1 months, respectively. Compared with the other two cohorts, the survival time was significantly prolonged, which means that patients treated with pemigatinib had very good results.
In terms of safety, the incidence of adverse events of pemigatinib is similar to that of other selective FGFR inhibitors. On the whole, it is well tolerated and the toxic and side effects are controllable.
Abnormal activation of fgf/fgfr pathway can enable tumor cells to maintain growth in a "self-sufficient" manner, so FGFRs has become a new target for tumor therapy. Clinical data show that 13%~20% of patients with cholangiocarcinoma carry FGFR2 fusion.
Therefore, if patients with cholangiocarcinoma want to use pemigatinib for treatment, they first need to determine whether they carry FGFR2 gene mutation. For details of cancer friends who want to carry out gene testing, they can consult the cancer free home medical department. If they have done gene testing, they can submit the test report to the medical department for condition evaluation.
The first FGFR targeted drug approved for marketing: erdatinib
Balversa (erdafitinib) is a fgfr1~4 tyrosine kinase inhibitor with antitumor activity. It is the first FDA approved oral FGFR inhibitor. In April, 2019, FDA accelerated the approval of erdatinib for the treatment of adult patients with locally advanced or metastatic bladder cancer after platinum chemotherapy with FGFR3 or FGFR2 mutations, including patients within 12 months of neoadjuvant or adjuvant platinum chemotherapy.
Its approval was based on the results of a phase II clinical trial blc2001. Among them, 87 patients with bladder cancer with advanced FGFR gene mutation received targeted therapy, and the objective remission rate was 32.2%: complete remission rate was 2.3% + partial remission rate was 29.9%. At present, the updated data of the trial show that after 99 patients with bladder cancer with advanced FGFR gene mutation received targeted therapy, the objective remission rate was 40%: the complete remission rate was 3% + the partial remission rate was 37%.
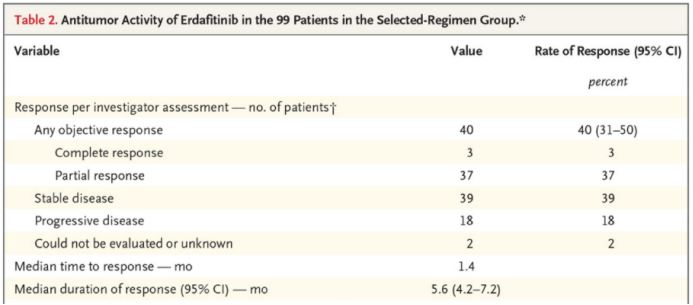
Updated data of blc2001 clinical trial
The objective remission rate was 59% in 22 patients who had previously received immunotherapy after the updated data of blc2001 clinical trial. The median progression free survival was 5.5 months and the median overall survival was 13.8 months.
For erdatinib, another phase I clinical trial involving 187 patients with advanced solid tumors is also noteworthy: 26 patients with bladder cancer, with an effective rate of 46.2%; The median duration of efficacy maintenance was 5.6 months; In 11 patients with cholangiocarcinoma, the effective rate was 27.3%, and the median time to maintain the efficacy was 11.4 months. In addition to the approved clinical indications, the clinical indications under study include non-small cell lung cancer, gastric cancer, esophageal cancer, cholangiocarcinoma, multiple myeloma, etc.
The third FGFR targeted drug: infigratinib
Infigratinib (bgj398, truseltiq) is one of the FGFR targeted drugs that first started clinical trials. It can be regarded as a veteran targeted drug in the industry. On May 29, 2021, FDA announced that infigratinib (truseltiq) was approved to be listed for the treatment of patients with locally advanced or metastatic cholangiocarcinoma with FGFR2 fusion and rearrangement mutations who had been treated.
This approval was based on the data of a phase 2 clinical study. A total of 108 patients with FGFR2 positive advanced cholangiocarcinoma who had received at least one treatment in the past were included.
The results showed that the objective response rate was 23% and the median progression free survival time was 7.3 months in patients treated with infigratinib; Among the patients who responded, 32% had remission lasting more than 6 months, and the median duration of remission was 5.0 months. The median overall survival was 12.2 months. Infigratinib was safe and well tolerated in this study.
Extension: infigratinib was used to treat lung squamous cell carcinoma, and the disease control rate reached 47.6%
Infigratinib is a small molecule inhibitor targeting fgfr1~3. In its phase I clinical trial, 21 patients with lung squamous cell carcinoma with FGFR1 amplification were included. The treatment dose was 100mg/d or higher. The curative effect of 17 patients was assessable, including 3 patients with partial remission and 7 patients with stable disease; The disease control rate was 47.6%.
FGFR targeted drugs under development
In addition to the above three listed drugs, there are more than 10 candidate drugs of Pan FGFR inhibitors at different stages of clinical development.
Second generation FGFR targeted drug: futibatinib
Futibatinib (tas-120) is a second generation FGFR targeted drug, which is used to treat cholangiocarcinoma patients who are resistant to the first generation FGFR inhibitor. In 2018, the US FDA granted futibatinib orphan drug qualification (odd) for the treatment of cholangiocarcinoma. On April 2, 2021, futibatinib was approved by FDA as a breakthrough therapy for the treatment of locally advanced or metastatic cholangiocarcinoma.
On March 30, 2022, it was reported that FDA had accepted the application for new drug (NDA) of futibatinib for locally advanced or metastatic cholangiocarcinoma that had previously been treated and carried FGFR2 fusion (including gene fusion). At the same time, FDA has also granted the application the priority review qualification, and it is expected that the results will be released by September 30, 2022 at the latest.
This new drug application is based on the positive results of the key phase 2B clinical study foenix-cca2. A total of 103 patients with locally advanced or metastatic intrahepatic cholangiocarcinoma were included as research subjects. The results published at the AACR conference in April 2021 showed that the objective remission rate of futibatinib treatment was 41.7%. The median duration of remission was 9.7 months, 72% of the patients' remission time was ≥ 6 months, the disease control rate was 82.5%, the median progression free survival was 9.0 months, the median overall survival was 21.7 months, and 72% of the patients were still alive at 12 months.
Effective for liver cancer, FGFR targeted new drug: blu-554
Blu-554 is a powerful and selective small molecule inhibitor of FGFR4. Phase I clinical data showed that among 38 patients with liver cancer with positive immunohistochemical staining for Fgf19, 6 patients had significantly reduced tumor size, with an objective remission rate of 16%, 26 patients (68%) had stable disease, and 18 patients (49%) had reduced tumor load. The maximum tumor reduction (measured by target lesion reduction) in 38 patients with fgf19ihc+ is shown in the figure.
Blu-554 treatment data
The above data show that blu-554 has initially realized the original intention of "divide and cure" for liver cancer, and is of great significance for the treatment of liver cancer.
Emerging pan FGFR inhibitor: derazantinib
Derazantinib is a potent, oral, targeted, pan FGFR (fibroblast growth factor receptor) inhibitor with strong activity on FGFR1, 2 and 3. At present, it is being developed to treat intrahepatic cholangiocarcinoma (ICCA) and other tumor types with high frequency of FGFR mutations.
The latest interim results of the phase II study cohort 2 of the targeted anticancer drug derazantinib in the treatment of cholangiocarcinoma fides-01 were published at the 2022 American Society of clinical oncology Gastrointestinal Cancer Symposium (ASCO GI). The group included patients with locally advanced or metastatic intrahepatic cholangiocarcinoma (ICCA) carrying FGFR2 gene mutation or amplification.
As of August 31, 2021, the deadline for interim analysis, 28 patients received treatment, of which 23 patients were eligible for efficacy evaluation. The median follow-up time was 5.2 months. According to the investigator's assessment, the disease control rate (DCR) was 74%, including 2 patients with confirmed objective remission and 15 patients with stable disease. Tumor shrinkage was observed in most patients. The median progression free survival (PFS) was 7.3 months.
According to the data of phase II study cohort 1 of fides-01 published in May 2021 (including 103 patients with gfr2 positive intrahepatic cholangiocarcinoma who have received at least one chemotherapy regimen), the objective response rate was 21.4%, the disease control rate was 74.8%, and the median progression free survival was 7.8 months.
For gastric cancer and gastroesophageal junction cancer: bemarituzumab
Bemarituzumab is the first antibody of its kind. As a tumor targeting therapy against fgfr2b overexpression, it is being developed for gastric cancer and gastroesophageal junction cancer. About 30% of patients with HER2 negative gastroesophageal carcinoma overexpress fibroblast growth factor receptor 2B (fgfr2b). Bemarituzumab (bema) is the first and only research therapy for fgfr2b+ tumors.
On April 20, 2021, FDA awarded the title of bemarituzumab breakthrough therapy developed by Amgen for the first-line treatment of fgfr2b positive and HER2 negative patients with locally advanced or metastatic gastric and gastroesophageal junction cancer in combination with the improved FOLFOX6 protocol (mfolfox6).
A total of 155 patients with gastric cancer / gastroesophageal junction cancer who had not been systematically treated were enrolled in the fifth trial, and were divided into bemarituzumab combined with mfolfox6 group and mfolfox6 group. The median progression free survival was 9.5 months and the 1-year progression free survival rate was 52.5% in the combined treatment group; The median progression free survival in the control group was 7.4 months, and the 1-year progression free survival rate was 33.8%.
Treatment of cholangiocarcinoma and solid tumors: icp-192
Icp-192 is a class 1 innovative drug with global independent intellectual property rights. It is a small molecule pan FGFR inhibitor with high selectivity that can be used to treat a variety of solid tumors. At present, it is carrying out a number of clinical studies in China and the United States.
On June 10, 2021, gunagratinib (icp-192) was granted the orphan drug qualification by the US FDA for the treatment of cholangiocarcinoma.
At the 2021 ASCO annual meeting, relevant researchers presented the latest clinical data of the pan FGFR inhibitor icp-192 in solid tumor patients. As of February 2021, 30 patients had received icp-192 treatment. Among the 12 patients with fgf/fgfr gene mutations who completed at least one tumor evaluation, the overall objective response rate (ORR) was 33.3%, including 1 complete response (CR), 3 partial response (PR) and 7 stable disease (SD). The disease control rate (DCR) was 91.7%. In addition, icp-192 performed well in safety and tolerability, and did not reach the maximum tolerable dose (MTD).
In addition to the drugs mentioned above, there are many FGFR inhibitors under development in clinical trials. In addition to pan FGFR inhibitors, enterprises have begun to deploy FGFR4 inhibitors. Moreover, the indications of FGFR inhibitors are more extensive. In addition to cholangiocarcinoma and urothelial carcinoma, they have expanded to hepatocellular carcinoma, gastric and gastroesophageal junction carcinoma, mesothelioma and so on.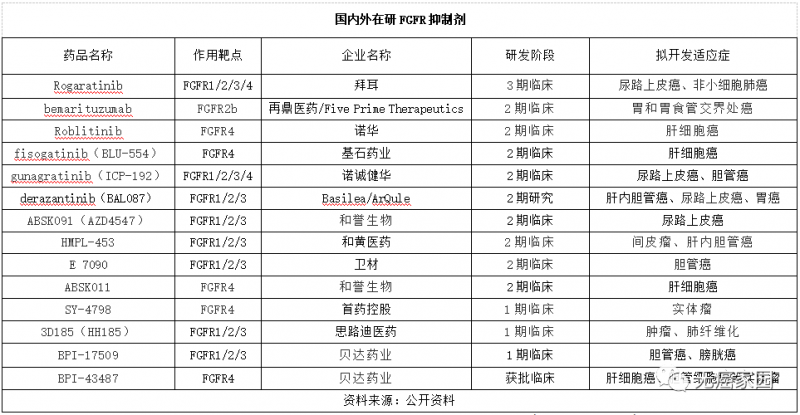
FGFR inhibitors developed at home and abroad
Xiaobian has something to say
The overall annual incidence of FGFR related solid tumors in the world increased from 4.4 million in 2016 to 4.9 million in 2020, with a CAGR of 3.0%, and is expected to increase to 6.8 million in 2035. This figure in China will reach 1.4 million in 2020, with a compound annual growth rate of 2.6% from 2016 to 2020. It is expected to reach about 1.9 million in 2035.
The abnormal expression of FGFR in human tumors is closely related to tumor development, prognosis and drug resistance. Therefore, the development of anti-tumor drugs targeting FGFR can provide a new and effective strategy for its related cancer treatment.
With the in-depth development of clinical research, scientists also found that patients with a higher degree of FGFR gene amplification had a better response to FGFR inhibitors. This shows that FGFR can not only be used as a target, but also as a therapeutic effect prediction index, and the market space is huge.
Reference:
1 https://pubmed.ncbi.nlm.nih.gov/26373574/
2 https://www.onclive.com/view/fda-approves-infigratinib-for-cholangiocarcinoma
3 https://www.onclive.com/view/derazantinib-continues-to-elicit-encouraging-responses-in-fgfr2-gene-fusion-positive-intrahepatic-cholangiocarcinoma
Article source:
http://www.globecancer.com/azzx/show.php?itemid=15109