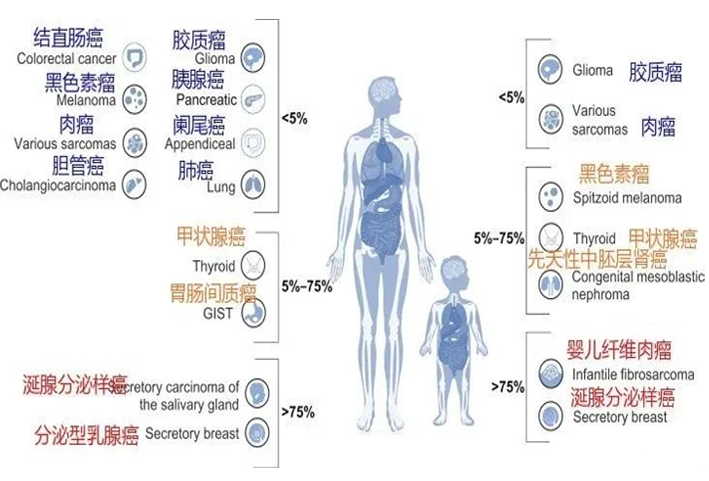
It has always been the expectation of all cancer patients to use and afford good drugs. In recent years, through the national medical insurance negotiation and other means, the anti-tumor drugs known as "life-saving drugs" have been greatly reduced, and the accessibility of patients has been continuously improved. So, what is the latest policy of Medicare on anti-cancer drugs and treatment? In this regard, the National Medical Insurance Bureau replied to the suggestions and proposals of the representatives and members of the two sessions in September.
Commonly used anti-tumor drugs have been included in medical insurance Category a reimbursement catalogue is not yet mature
In its reply to recommendation No. 1924 of the fourth session of the 13th National People's Congress, the National Medical Insurance Bureau introduced that in recent years, governments at all levels have continued to increase investment, supported the strengthening of the triple security functions of basic medical insurance, serious illness insurance and medical assistance, and steadily improved the basic medical security level of insured residents, including cancer patients. First, increase investment in medical insurance for urban and rural residents. Taking 2020 as an example, governments at all levels will allocate a total of 580 billion yuan of subsidy funds. Second, support serious illness insurance for urban and rural residents. In 2018 and 2019, half of the new per capita subsidy for medical insurance for urban and rural residents will be used for serious illness insurance. Third, support medical assistance. In 2020, the central government will allocate 32 billion yuan of medical assistance subsidies to support basic medical assistance.
The national medical insurance bureau pointed out that through the adjustment of the medical insurance drug catalogue, most of the anti-tumor drugs commonly used in clinic have been included in the payment scope of basic medical insurance, which can meet the basic medical needs of residents. Through drug price negotiation and centralized procurement, actively guide the price reduction of anti-tumor drugs, and further reduce the drug burden of cancer patients.
For promoting the inclusion of tumor targeted drugs in the category a reimbursement catalogue of medical insurance, the National Medical Insurance Bureau said that China's current financing level of basic medical insurance is low. In 2020, the per capita medical insurance for urban and rural residents will be only about 800 yuan, with limited guarantee capacity. Anti-tumor drugs are generally expensive, so the conditions for their inclusion in the management of class a drugs in medical insurance are not mature. In the future, the dynamic adjustment mechanism of the medical insurance catalogue will be improved, and more qualified antitumor drugs will be included in the reimbursement catalogue according to procedures.

In the past three years, 433 new drugs have been added to the medical insurance catalogue, most of which are new anti-tumor drugs
In its reply to proposal No. 2836 of the fourth session of the 13th National Committee of the Chinese people's Political Consultative Conference, the National Medical Insurance Bureau introduced that since its establishment, the National Medical Insurance Bureau has established a dynamic adjustment mechanism for the medical insurance drug catalog, which has been shortened from one adjustment in eight years to dynamic adjustment every year. The catalog adjustment cycle has been greatly shortened. Many innovative drugs can apply to enter the catalog in the year when they are approved for listing.
According to the National Medical Insurance Bureau, the Interim Measures for the administration of drugs for basic medical insurance (for Trial Implementation) issued in 2020 clearly stipulates that all drugs outside the catalogue shall be subject to the declaration system, pharmaceutical enterprises shall declare independently, and the drugs that meet the declaration conditions shall enter the evaluation link according to the procedures. From the perspective of application scope, catalog access is increasingly focused on new drugs. Taking 2021 as an example, a key scope of application for drugs outside the catalogue is the newly listed drugs since 2016.
According to the National Medical Insurance Bureau, after three consecutive years of adjustment, a total of 433 new drugs have entered the national medical insurance catalogue. Many of them are new anti-tumor drugs approved by the State Food and Drug Administration in recent years, especially the drugs supported by the major scientific and technological projects of "major new drug creation", most of which have been included in the national drug catalogue. For example, China's original anti-tumor new drug sidaniline, the world's first small molecule anti angiogenesis targeted drug apatinib for the treatment of advanced gastric cancer, China's first small molecule targeted anti-tumor new drug ektinib hydrochloride with independent intellectual property rights, PD-1 drugs cindilimab and carrelizumab have been included in the national medical insurance drug catalogue through medical insurance access negotiations. New antitumor drugs such as rituximab, sorafenib, gefitinib and fluvestrant have been included in the scope of medical insurance payment.
Some immune drugs are included in medical insurance through negotiation
In its reply to proposal No. 3657 of the fourth session of the 13th National Committee of the Chinese people's Political Consultative Conference, the National Medical Insurance Bureau introduced that at present, the total number of Western and Chinese patent medicines in the 2020 national medical insurance drug catalogue is 2800. Through the medical insurance access negotiation, the price reduction of expensive immune drugs such as oshtinib, trastuzumab and olapari will be included in the scope of medical insurance payment, It effectively reduces the burden of patients.
In terms of diagnosis and treatment items, the national level adopted the exclusion method, which stipulated the diagnosis and treatment items for which the basic medical insurance did not pay and paid part of the expenses, and did not exclude the diagnosis and treatment items related to immune testing. The medical insurance departments of all provinces (regions and cities) have determined the payment scope of diagnosis and treatment projects in the province according to the actual situation of medical technology development and the operation of medical insurance funds in the region. Some areas have included some immune testing items in the scope of medical insurance payment.
Source: People's daily
http://health.people.com.cn/n1/2021/0924/c14739-32235813.html