The International Association for the study of lung cancer (IASLC) is the only global organization dedicated to lung cancer research. The World Lung Cancer Congress (wclc) is the world's largest conference sponsored by IASLC and dedicated to lung cancer and other malignant tumors of the chest. From August 6 to 9 this year, the 2022 wclc is being held in Vienna. In addition to reporting the efficacy of EGFR and met targeted drugs at the meeting, many breakthrough new developments in KRAS G12 mutations that were difficult to target therapy in the past were also reported.
KRAS G12C mutation
KRAS mutations are common driver gene mutations in non-small cell lung cancer (NSCLC), most of which occur in lung adenocarcinoma. KRAS mutations account for about 10% of lung adenocarcinoma in China and about 30% in Western populations. The highest proportion of different KRAS mutation subtypes is G12C (40%) [1]. In recent years, KRAS G12C targeted drugs have shown good efficacy and safety in clinical studies. The first oral KRAS G12C targeted drug sotorasib (sotorasib) was approved by the US FDA last year, and the second KRAS G12C targeted drug adagrasib was also submitted to the US FDA at the beginning of this year. This year, wclc not only reported the efficacy of combination therapy with existing KRAS targeted drugs, but also reported the efficacy of new drugs including domestic drugs.
Sotorasib combined with targeted new drugs, the remission rate surged to 75%
The 2-year follow-up data of sotorasib released at the 2022 AACR conference showed that with the expansion of the patient evaluation population, the objective response rate (ORR) reached 40.7%, and its efficacy was significantly improved compared with chemotherapy. However, compared with the 60% - 70% orr of EGFR targeted drugs, there is still room for improvement. Combining with other targeted drugs is a feasible strategy to improve the orr.
The conference reported a dose escalation clinical study of sotorasib combined with the small molecule SHP2 inhibitor rmc-4630 in the treatment of KRAS G12C mutant NSCLC and other solid tumors [2].
Twenty one patients were included in the study, including 11 patients with NSCLC, 6 patients with colon cancer and 4 patients with other solid tumors. Ten patients had previously received KRAS G12C targeted therapy, including 8 patients treated with sotorasib and 2 patients treated with adagrasib.
Results, of the 11 NSCLC patients, 3 (27%) had confirmed partial remission (PR), of which 2 were still in remission at the time of data cutoff; 7 cases (64%) had disease control (partial remission + stable disease). Among the 4 NSCLC patients who received the two highest doses of rmc-4630 combined with sotorasib without KARs targeted therapy, 3 (75%) patients had confirmed PR, 2 of them were still in continuous remission, and 4 (100%) patients had controlled disease.
One NSCLC patient progressed after previous sotorasib treatment. After 5.5 months of treatment with rmc-4630, the tumor shrank by more than 30%, and the disease progressed after 6.9 months of treatment; PR was confirmed in one patient with ovarian cancer, and tumor burden was reduced by 81%; Disease control was achieved in 5 / 6 colon cancer patients, including a 26% reduction in tumor burden in 1 patient.
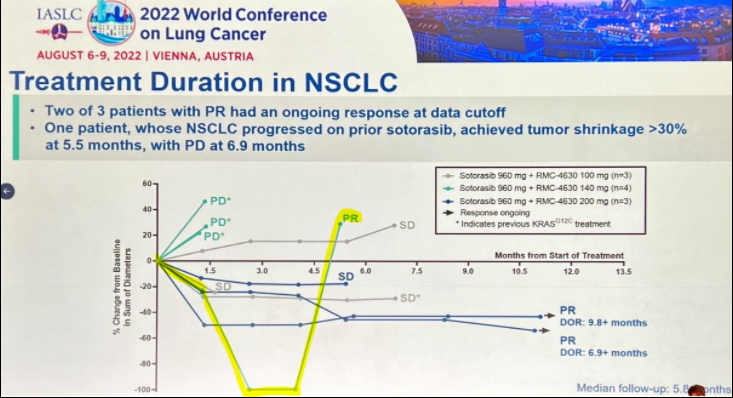
In one patient who was previously treated with sotorasib, the tumor shrank again after the combination of rmc-4630, and then the disease progressed [ls1] (yellow curve) [2]
In terms of safety, none of the patients had grade ≥ 4 treatment-related adverse events (traes). 71% of patients had any grade of traes: the most common were peripheral edema and local edema (33%), diarrhea (29%) and fatigue (14%). There were 6 patients (29%) with grade 3 traes (2 cases with diarrhea; 1 case with ascites, elevated ast, colitis, dyspnea, hypertension and pleural effusion). Two patients had traes leading to the discontinuation of rmc-4630 (one with diarrhea and one with ascites), and one patient had traes leading to the discontinuation of rmc-4630 and sotorasib (AST elevation).
Sotorasib combined with immunotherapy has increased hepatotoxicity, and the efficacy needs further study
The meeting reported the first results of sotorasib combined immunotherapy. The 1b study named codebreak 100 / 101 explored the efficacy and safety of sotorasib combined with pablizumab (drug K) or atilizumab (drug t) in the treatment of KRAS G12C mutant NSCLC [3]. The study was divided into 12 dose exploration cohorts. Patients received different doses of sotorasib (120-960mg once a day) combined with intravenous infusion of 1200mg of T drug or 200mg of K drug. Immunotherapeutic drugs were administered once every 3 weeks until intolerable or disease progression. Half of the cohort was the introduction cohort, and these patients received sotorasib monotherapy for 21 or 42 days before the first dose of immunotherapy drugs, and then received t or K drugs combined with sotorasib. The other half was a synchronous cohort, and sotorasib was used in combination with T or K drugs at the beginning.
58 patients were included in the study, with a median follow-up of 12.8 months (range: 1.6, 29.9). The median of previous treatment lines was 1 (range 0-7); 67% of the patients had previously received immunotherapy. The most common grade 3-4 treatment-related adverse events (traes) were elevated ALT and AST. Among patients with grade 3-4 hepatotoxic trae, 22 (88%) of 25 patients first appeared outside the dose limiting toxicity assessment period, suggesting delayed toxicity, most of them were treated with corticosteroids, and 97% of the events were resolved. The incidence of hepatotoxicity did not differ between patients who did not receive immunotherapy and those who had received immunotherapy. No fatal trae occurred. Grade 3-4 traes and traes leading to treatment discontinuation occurred less frequently in the introduction cohort than in the synchronous cohort.
In all 12 cohorts, partial remission was confirmed in 17 of 58 patients (ORR 29%; 95%ci, 0-67). Among the 17 patients with remission, the median duration of remission (DOR) was 17.9 months (95%ci:1.5, 23.4). The median overall survival OS of all 58 patients was 15.7 months (95% ci:9.8, 17.8).
This study showed that the combination of sotorasib and immunotherapy resulted in a higher incidence of grade 3-4 trae than that of monotherapy, mainly due to elevated liver enzymes. Compared with the synchronous cohort, the introduction cohort showed long-lasting clinical efficacy, and the incidence of grade 3-4 trae was lower. Therefore, the ongoing dose expansion study uses the scheme of first introducing low-dose sotorasib and then combining low-dose sotorasib with K drug for the newly treated patients.
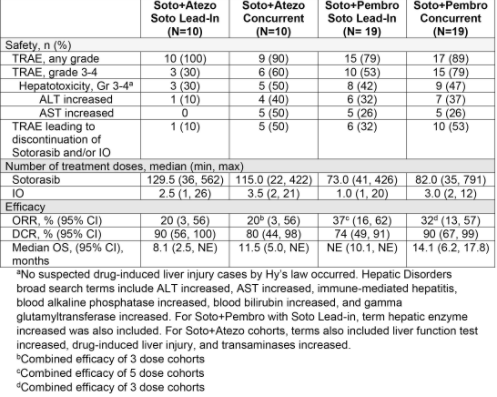
Figure 1 Summary of safety and efficacy of combination therapy [3]
Lead in is the introduction cohort, concert is the synchronization cohort, and IO is the immunotherapy
D-1553, a new KRAS targeting drug made in China, has a remission rate of nearly 40% and is also effective in brain metastasis
D-1553 is a new, efficient and oral KRAS G12C targeted drug independently developed by Yifang biology. At the conference, Professor Lu Shun reported the efficacy and safety of this drug in the phase I dose escalation study [4].
The study included 79 patients with KRAS G12C mutant NSCLC who had received systemic anticancer treatment with a median of 2 lines (95% ci:1-7), of which 42 patients (53.2%) received ≥ 2 lines of treatment. By the time of data cutoff, the median follow-up time was 21.7 weeks (95% ci:3-47).
Of all 79 patients, 53 patients (67.1%) were still receiving treatment, no dose limiting toxicity was reported, and the maximum tolerated dose was not reached. Treatment related adverse events (traes) occurred in 68 patients (86.1%), most of which were grade 1-2. The most common (≥ 20%) traes were ast elevation, ALT elevation γ- Elevated glutamyltransferase, elevated conjugated bilirubin, and anemia. Grade 5 trae was not reported.
73 patients in all dose groups in the study were able to evaluate tumor response, 29 patients were partial remission (PR), and 38 patients were stable in disease (SD); The Orr and disease control rate (DCR) were 39.7% (29 / 73) and 91.8% (67 / 73), respectively. Three patients had measurable brain metastases at baseline, and the results were 1 PR and 2 SD in these patients. The median duration of remission (DOR) has not been reached, but 25 (86.2%) of the 29 patients with remission were still in remission, and 14 of them had dor ≥ 12 weeks. Progression free survival has not been achieved, and 57 (78.1%) patients have not progressed.
The new KRAS targeting drug gdc-6036 has a remission rate of nearly 40%
Gdc-6036 is a new KRAS targeting drug. The conference reported the safety and efficacy of dose escalation group (NSCLC) and dose expansion group (solid tumor) in its phase I study [5].
Thirty seven treated patients with KRAS G12C mutant NSCLC were included in the study, including 27 in the dose escalation group and 10 in the dose expansion group.
All solid tumor patients in the dose escalation group were unreported dose limiting toxicity. Among all NSCLC patients, the median time to study treatment was 3.5 months (range: 0-9.7 months), and the median cumulative dose intensity was 99%. 17 NSCLC patients (46%) stopped the study treatment, including 12 patients due to disease progression, 3 patients due to doctor's decision, 1 patient due to withdrawal of the agreement, and 1 patient due to adverse events of diarrhea.
Nausea, vomiting, diarrhea and fatigue were reported in ≥ 20% of patients. Grade 3 adverse events related to gdc-6036 were ≥ 2 cases (5%), including ALT / AST elevation and diarrhea; There were no grade 4-5 adverse events related to gdc-6036.
Adverse events were controlled by auxiliary measures. For gdc-6036 related adverse events, 13 (35%) patients required dose adjustment. Among NSCLC patients in each dose group, the unconfirmed orr was 43% (15 / 37) and the confirmed orr was 37% (13 / 37).
EGFR mutation
EGFR mutation is the most common driver gene mutation in Chinese lung adenocarcinoma patients. Targeted therapy has greatly prolonged the survival of patients with EGFR mutations. However, drug resistance is always a difficult problem for patients. For this reason, this conference has the special topic of "overcoming EGFR inhibitor resistance", which reports the new progress including the fourth generation targeted drugs. We will report the fourth generation targeted drugs separately in a special article. Here, we will first introduce the new progress of other schemes to overcome drug resistance.
The four drugs cooperated to control drug resistance, and the remission rate was as high as 50%
Amivantamab is a dual target monoclonal antibody against EGFR / met. Previous studies showed that amivantamab combined with the third generation EGFR targeting drug lazetinib has a certain effect on drug-resistant refractory EGFR mutation patients. This conference reported the efficacy and safety of amivantamab + lazetinib + pemetrexed + carboplatin four drug regimen (LACP regimen) in the treatment of drug-resistant refractory EGFR mutations [6]. The chemotherapy drug carboplatin in this scheme is only used for 4 cycles.
Twenty patients with refractory EGFR mutations were included in the study. The median number of previously received treatment regimens was 2 (range, 1-3). Previously used targeted drugs included ositinib (n = 14), gefitinib (n = 3) and afatinib (n = 3). Up to now, in the follow-up of at least 3 months, 10 patients have been confirmed as partial remission (PR), 7 patients have stable disease (SD), and 3 patients have disease progression. The most common treatment-related adverse events were infusion related reactions (73.3%), neutropenia (66.7%), rash (46.7%), thrombocytopenia (40.0%), fatigue and nausea (33.3% each). Five patients discontinued treatment, two because of serious adverse events related to chemotherapy, and three because of disease progression.
Tepotinib is effective in treating the jump mutation of met exon 14 (metex14) and brain transformation
The incidence of metex14 jump mutation in non-small cell lung cancer is 3% ~ 4%. Targeted drugs that act on metex14 jump mutations include crizotinib, camatinib, tepotinib, sevotinib, etc. Currently, sevotinib has been listed in China, while camatinib and tepotinib are urgently needed for clinical application in specific medical institutions in the international medical tourism pilot area of Boao Music City, Hainan. This meeting reported the latest efficacy data of tepotinib vision study [7].
The phase II vision study of tepotinib includes group A (preliminary analysis) and group C (confirmation analysis). The results of group A have been reported previously. The conference reported the combined analysis results of group C and group A + C.
The median age of patients in group C (n = 161) was 71.0 years (range 42-91), 46.6% were male, 54.0% were white, 42.2% were Asian, 43.5% had smoking history, 75.2% were adenocarcinoma, 74.5% ECoG PS score was 1, and 59.0% were newly treated (1L). In group C, the orr was 54.7% (46.6, 62.5), the median duration of remission (mdor) was 20.8 months (12.6, not assessable), and the median progression free survival (MPFs) was 13.8 months (10.4, not assessable). The curative effects of different treatment lines are stable and lasting. See the following table for details:
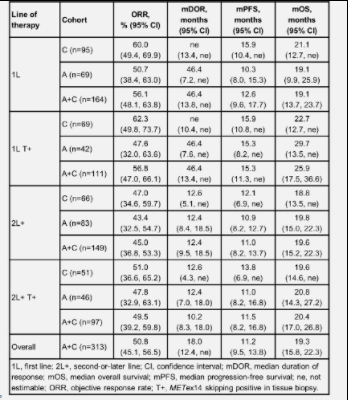
Fig. 2 summary of efficacy of tepotinib [7], 1L represents first-line treatment (initial treatment); 2L represents second-line treatment; T + represents the positive detection of metex14 jump mutation in tumor tissue samples; Orr represents objective response rate; Mdor represents the median duration of remission; MPFs represents median progression free survival; MOS represents median overall survival
In group A + C, 43 patients with brain metastases could be evaluated (1L, n = 23; 2L +, n = 20); Thirty (69.8%) patients had received brain radiotherapy or surgery. The intracranial (I) disease control rate was 88.4% (74.9, 96.1), the IPFs was 20.9 months (5.7, not evaluable), the iorr was 66.7% (38.4, 88.2), and the idor was not evaluable (0.9, NE) in patients with only target lesions (n = 15). In group A + C, 91.7% of patients (≥ grade 3 34.2%) had treatment-related adverse events (trae); Including (≥ 15%) peripheral edema (any grade / ≥ 3: 66.5% / 10.9%), nausea (23.3% / 0.6%), hypoalbuminemia (23.0% / 3.2%), diarrhea (22.4% / 0.3%), and elevated blood creatinine (21.7% / 0.6%). 14.7% of the patients were permanently discontinued due to trae.
Reference:
1. Nong Jingying, Li Xiaoxue, Yao Shuyang, et al Progress in biology and treatment of KRAS mutant non-small cell lung cancer [J]. Cancer research and clinical, 2021, 33 (1): 69-73
DOI: 10.3760/cma.j.cn115355-20200909-00514.
2. Bob T. Li, et al. OA03.03 - Sotorasib in Combination with RMC-4630, a SHP2 Inhibitor, in KRAS p.G12C-Mutated NSCLC and Other Solid Tumors. presented at WCLC 2022.
3. Gerald Falchook et al. OA03.06 - CodeBreaK 100/101: First Report of Safety/Efficacy of Sotorasib in Combination with Pembrolizumab or Atezolizumab in Advanced KRAS p.G12C NSCLC. Presented at WCLC 2022.
4. Hong Jian, et al. OA03.07 - Safety and Efficacy of D-1553 in Patients with KRAS G12C Mutated Non-Small Cell Lung Cancer: A Phase 1 Trial. presented at WCLC 2022.
5. Manish Patel et al. OA03.04 - Phase I A Study to Evaluate GDC-6036 Monotherapy in Patients with Non-small Cell Lung Cancer (NSCLC) with KRAS G12C Mutation. presented at WCLC 2022.
6. Se-Hoon Lee et al. MA07.04 - Amivantamab and Lazertinib in Combination With Platinum-Based Chemotherapy in Relapsed/Refractory EGFR-mutant NSCLC. Presented at WCLC 2022.
7. Marina Chiara Garassino et al. OA03.05 - Tepotinib in Patients with MET Exon 14 (METex14) Skipping NSCLC: Primary Analysis of the Confirmatory VISION Cohort C. Presented at WCLC 2022.
Source:
https://www.cn-healthcare.com/articlewm/20220810/content-1415759.html