Chinese made targeted drug KRAS G12C inhibitor zg19018 has been approved for clinical trials in the United States, and more KRAS G12C clinical trials are under recruitment
On January 23, Zejing pharmaceutical announced that the clinical trial application of zg19018 tablets under development was approved by the U.S. Food and Drug Administration (FDA) for the treatment of advanced malignant solid tumors with KRAS G12C mutation.
Zg19018 is a selective covalent inhibitor of KRAS G12C independently developed by the company, with global intellectual property rights. At present, only one drug with similar mechanism has been approved to market all over the world. In addition, monotherapy or combination of KRAS G12C inhibitors also showed good efficacy and safety in advanced KRAS, G12C, and advanced pancreatic cancer.
The results of preclinical studies showed that zg19018 had significant pharmacodynamic effects on inhibiting the growth and cell proliferation of KRAS G12C mutant tumor, had long drug half-life and high oral bioavailability, and had high drug concentration in tumor and brain tissue. Zg19018 is expected to become an innovative drug for the treatment of KRAS G12C mutant tumors.
The nail households of targeted therapy have been overcome, and a number of KRAS new drugs have blowout
To say that the most malignant, the most difficult to deal with and the most terrible carcinogenic mutation, it must be KRAS gene mutation! As a "nail household" in targeted therapy, almost all targeted drugs have failed in front of it in recent 40 years.
According to medical literature, KRAS is one of the most common oncogenes in solid tumors. About 30% of the tumors are KRAS mutations, including 90% of pancreatic cancer, 30%~40% of colon cancer and 15%~20% of lung cancer. However, there are few KRAS targeted drugs, and KRAS once became the most difficult mutation without drugs.
Among KRAS gene mutations, 97% occurred in amino acid residues 12 or 13, including G12C, G12D, g13d, etc., and G12C mutation was the most common. The KRAS G12C mutation occurred in 14% of lung adenocarcinoma (the most common subtype of NSCLC), 4% of colorectal cancer and 2% of pancreatic cancer.
Globally, there are more than 100000 newly diagnosed KRAS G12C mutant cancer patients every year. Patients with this mutation have a poor prognosis and are prone to drug resistance to standard therapy. Once chemotherapy or immunotherapy fails, the treatment options are very limited.
As the "most difficult target", for KRAS G12C mutation, there is only lumakras (sotorasib, amg510) approved by FDA on May 28. This is currently the only targeted drug approved for KRAS mutation, also known as the milestone for scientists to break through KRAS "non patent medicine"!
It is gratifying that with the continuous efforts of medical researchers, the fortress of KRAS has finally been broken, a variety of new drugs have emerged, and many drugs under research have achieved early success in clinical research: adagrasib (mrtx849), jnj-74699157 (ars-3248), jab-3312, ly3499446 and pan KRAS inhibitor Bi 1701963.
The disease control rate reached 100%, and adagrassib and K drugs achieved great results in the first-line treatment of lung cancer
It is expected to become the second batch of tumor specific Ras inhibitors for colorectal cancer after the optimization of Mrs 84asitor9 and other solid tumors.
Adagrassib has a half-life of up to 24 hours and a wide tissue distribution, and can cross the blood-brain barrier, which helps to maximize the effectiveness of the drug. In June this year, the US FDA granted it breakthrough therapy recognition for the treatment of patients with treated non-small cell lung cancer carrying KRAS G12C mutation.
In addition to evaluating the efficacy of adagrassib as a monotherapy, researchers have also made breakthroughs in trying to combine other targeted therapies or immunotherapies, such as cetuximab, afatinib or pabolizumab.
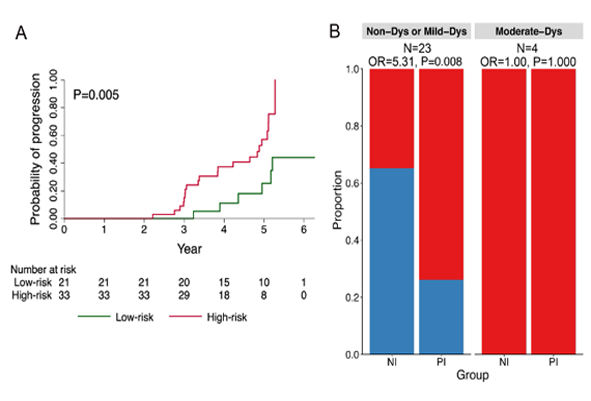
On November 8, 2021, in the third quarter financial report of mirati company, the preliminary results of phase 1b clinical trial of KRAS G12C inhibitor adagrasib combined with anti-PD-1 antibody pembrolizumab (pabolizumab) for first-line treatment of non-small cell lung cancer patients with KRAS G12C mutation were announced. The results showed that this combination therapy achieved 100% disease control rate (DCR) in 7 patients who could be evaluated.
In this clinical trial, as of October 1, four of the seven patients who could be evaluated had achieved confirmed partial remission, and another patient had a tumor reduction of 49%. He chose to undergo surgical resection before confirmation. The tumor reduction range of 7 patients was 37% ~ 92%. At a median follow-up of 9.9 months, 5 of the 7 patients were still receiving treatment. They have been treated for 8 to 11 months. Mirati said that this data supports the continued evaluation of the efficacy of this combination in phase 2 clinical trials.
What if you can't use amg510 and adagrassib? Chinese medicine d-1553 solves the urgent need
In view of the fact that amg510 has just been approved for listing, adagrasib is still in the clinical trial stage abroad, and Chinese people are in dire straits where one drug is difficult to find. China's pharmaceutical enterprises have also increased the process of new drug research and development on KRAS targets.
The good news is that d-1553, a new domestic drug, has officially started recruiting patients for all kinds of solid tumor patients with KRAS G12C mutation! Patients who wish to participate can consult the Department of cancer free home medicine for the trial.
Article link:
http://www.globecancer.com/azzx/show.php?itemid=14794