Viruses can cause cancer and also become "cancer busters".
At the 2022 Annual Meeting of the European Society of Medical Oncology (ESMO) held recently, British scientists submitted their research that genetic modification of herpes simplex virus can infect and destroy cancer cells, while activating the human immune system to treat cancer.
A phase I clinical trial involving 39 patients with late cancer showed that 1/4 of the subjects had significant efficacy, and the tumor stopped growing or shrinking. The tumor of one patient with salivary gland cancer completely disappeared and did not recur during the 15 month follow-up.
Photo caption: A recent photo of Krzysztof Wojkowski, a patient with salivary gland cancer/ The Telegraph
Kill cancer cells with viruses
According to comprehensive media reports, Krzysztof Wojkowski, a 39 year old construction worker, is the "lucky boy" mentioned above.
In May 2017, Kirishtov was diagnosed with salivary gland cancer. This is a tumor that starts with abnormal cell growth in the salivary gland and has a high incidence in oral and maxillofacial tumors.
After surgery and other comprehensive treatment, his condition was not controlled and the tumor continued to grow.
In 2020, Kirishtov joined the experimental group of Cancer Research Institute of the Royal Masden NHS Trust. The patients suffered from a variety of cancers, including skin cancer, esophageal cancer and head and neck cancer. In the same way, they are all in terminal cancer and receiving hospice care.
"This is my last hope." Kirishtov told the Daily Telegraph.
After enrollment, Kirishtov received virus injection once every two weeks, a total of 8 times. The initial dose is 10ml, in which the virus content reaches 1 × 106PFU/ml。 The total dose of the following 7 injections remained unchanged, and the virus content was 1 × 107PFU/ml。
The injected virus, named RP2, is a kind of oncolytic type I herpes simplex virus with enhanced efficacy and gene editing.
The gene editing process includes the insertion of granulocyte macrophage colony stimulating factor (GM-CSF), cytotoxic T-lymphocyte associated protein 4 (CTLA-4) antibody, and the codon optimized gibbon leukemia virus (GALV) glycoprotein (GP) gene, whose R transmembrane peptide is removed (GALV-GP-R -).
These modifications will attack cancer from two dimensions: first, the virus will infect cancer cells and normal cells. Since it cannot replicate in normal cells, it is harmless to them. But in cancer cells, the virus can replicate in large numbers until the cancer cells are broken and destroyed. Second, release GM-CSF, quickly activate the human immune system, find and kill cancer cells.
After treatment, Kirishtov improved significantly. A comprehensive examination showed that the cancer cells in his body were completely eradicated.
"Since joining the group, I have not detected cancer cells for 2 years. I return to work, share a good time with my family, and can do anything."
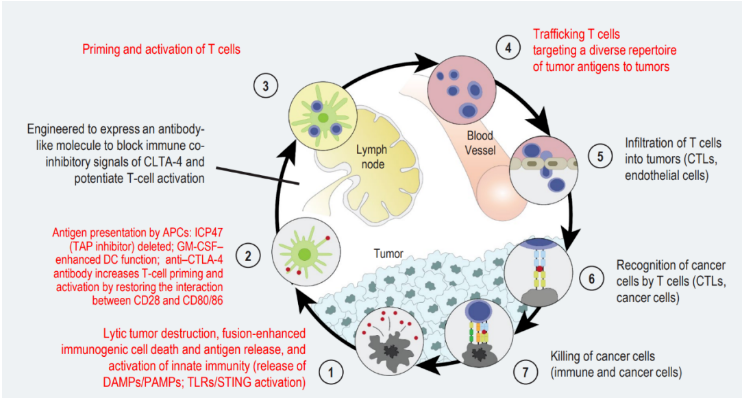
Photo caption: RP2 mechanism diagram/ ESMO
Safety research is effective
In the ESMO annual meeting held in September, Professor Kevin J. Harrington, the head of the aforementioned research and the cancer research institute of the Royal Masden NHS Trust, made an oral report.
According to his introduction, the first phase of the study, part 2a, has been completed, and the results are encouraging.
First, the biopsy before and after RP2 injection showed that the tumor immune microenvironment had positive changes. More immune cells, including CD8+T cells, can be detected in the injection area.
Second, among the 9 patients receiving RP2 single drug injection, 3 were observed to respond and the tumor was significantly reduced. In addition to Kirishtov, one patient with esophageal cancer accompanied by liver metastasis maintained the curative effect for more than 18 months, and the other one developed disease progress when responding for 15 months.
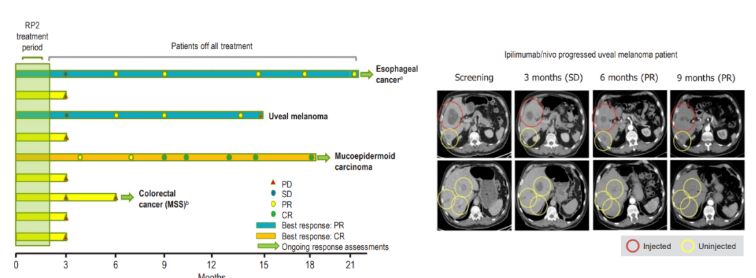
Photo caption: Effect diagram of RP2 single drug injection/ ESMO
Third, 30 subjects were treated with Navulizumab on the basis of virus injection. This is a monoclonal antibody targeting PD-1, which can regulate the immune system and urge T cells to play an immune monitoring role to eliminate cancer cells.
The results showed that the combination therapy showed good tolerance and lasting systemic reaction. In skin melanoma, uveal malignant melanoma (malignant intraocular tumor) and head and neck squamous cell carcinoma, the objective response rates were 44.4%, 25% and 33%, respectively.
All responding patients (7) had previously experienced immunotherapy failure. After this combined treatment, the continuous response of 6 patients was more than 425 days.
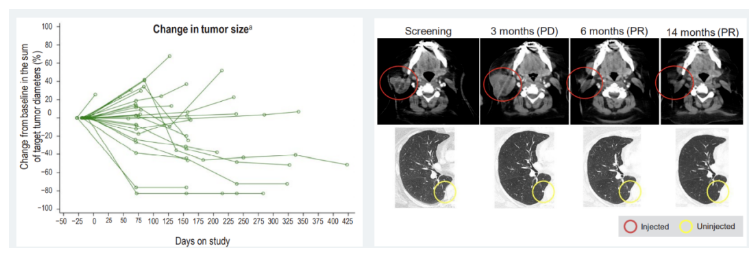
Photo caption: Effect diagram of RP2 single drug injection/ ESMO
Further analysis showed that the side effects of either single drug or combination treatment mainly included mild fever and fatigue, without medical intervention.
Kevin Harrington said: "Phase I clinical trial is an early safety trial, mainly aimed at testing the treatment safety. It is rare to see such a high treatment response rate."
He added that for some patients with late cancer, including those who do not respond to immunotherapy, the tumor lytic herpes simplex virus (HSV) after gene editing may be a new treatment option. In the future, the study will include more patients with solid tumors, including breast cancer, non-small cell lung cancer, etc. "I wonder if we can continue to see significant benefits as the number of patients increases."
It is not the first time for virus to cure cancer
The BBC reports that this is not the first time that scientists have used viruses to fight cancer.
In October 2015, T-VEC, an oncolytic virus product with herpes simplex virus type I as the carrier, was approved for marketing in the United States for the local treatment of the first recurrent and unresectable melanoma. The clinical research jointly participated by 64 authoritative research centers around the world showed that about 16.3% of patients who received T-VEC showed a lasting treatment response in more than 6 months. In contrast, only 2.1% of the control group showed persistent responses.
In September 2017, Cell magazine reported that T-VEC combined with PD-1 antibody drug treatment had a remission rate of 62% for melanoma, of which 33% was complete remission. This is much higher than the expected remission rate of PD-1 antibody alone.
In 2020, the monthly journal of the British Society for Cancer Immunotherapy published a review to analyze 97 open clinical trials related to oncolytic viruses from 2000 to 2020.
The article shows that there are many kinds of vectors for oncolytic drugs under research, including adenovirus, herpesvirus, cowpox virus and reovirus. As a new method, oncolytic virus can treat malignant tumors of different types and different stages of progression, and has made phased achievements in clinical research of various cancer treatments.
Kevin Harrington told the BBC that RP2 can be regarded as an enhanced or upgraded version of T-Vec, which can kill cancer cells more effectively.
Reference material:
[1]An open-label,multicenter,phase I study of RP2 as a single agent and in combination with nivolumab in patients with solid tumors:Safety,efficacy,and biomarker results. Annals of Oncology.(2022)33(suppl_7):S356-S409.10.1016/annonc/annonc1059
[2]Cancer-killing virus shows promise in patients.BBC
[3]‘Miracle’herpes treatment eradicates tumours in terminally-ill cancer patients. The Telegraph
Source:
https://www.cn-healthcare.com/article/20221004/content-573612.html