Recently, new progress has been made in the "collection of biologically similar drugs", which has been concerned for a long time.
Recently, when the National Medical Insurance Bureau responded to a number of proposals on its official website, it responded to the proposal of Zhu Yilong, member of the CPPCC National Committee, on "introducing an evaluation mechanism for the access of new innovative drugs" (Medical Insurance Letter [2022] No. 189), saying that it would constantly improve the rules for the centralized purchase of biological drugs based on the existing successful experience and the characteristics of biological drugs. This response is also seen by the industry as a signal to speed up the collection of biological drugs.

Reply of the State Medical Insurance Bureau to the proposal of Zhu Yilong, member of the National Committee of the Chinese People's Political Consultative Conference (screenshot source: the State Medical Insurance Bureau)
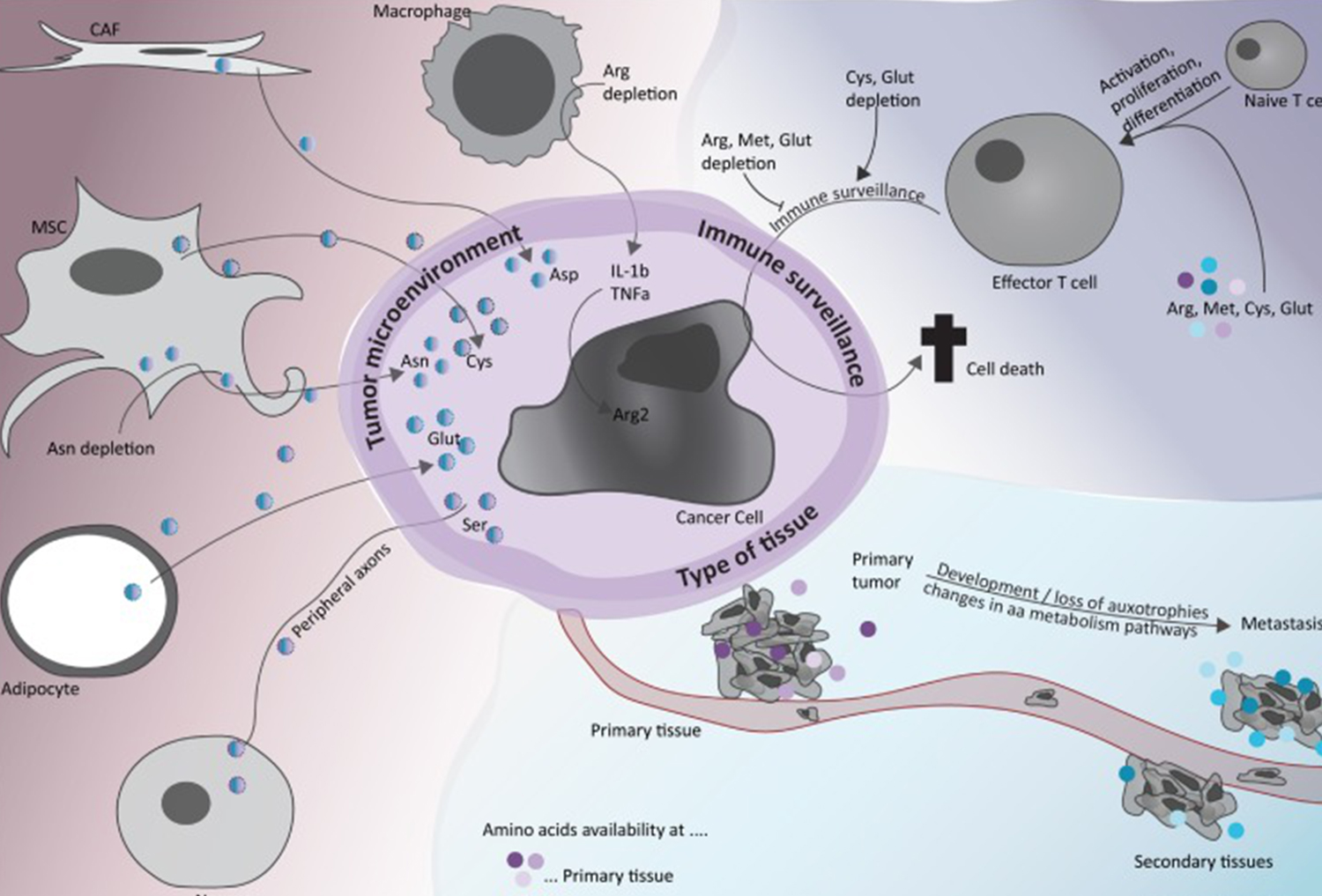
Public information shows that the so-called biological drugs refer to the drugs prepared by biosynthesis, including vaccines, blood and blood components, somatic cells, gene therapy, tissues and recombinant therapeutic proteins; In China, biosimilar (a kind of biological drug similar to the approved original biological drug) is the focus of many enterprises.
The reporter from Nandu Bay Finance Agency learned that, unlike conventional chemical drugs, it is difficult to select the reference and consistency evaluation of the original research drugs due to the complex product structure of biological analogues. In addition, the clinical use habits and patient compliance also affect the use effect of biological analogues, so it is difficult to carry out centralized procurement of such drugs in quantity.
However, in February this year, the relevant person in charge of the National Health Insurance Bureau introduced that the future drug collection will be carried out in three sectors: chemical medicine, Chinese patent medicine and biological medicine. This means that biologically similar drugs have been listed as the target of intensive procurement with quantity.
In fact, there has been a drill for the centralized procurement of biologically similar drugs before. The reporter from the Nandu Bay Finance Agency noticed that the sixth batch of national centralized procurement of drugs at the end of last year was a special insulin workshop, which was regarded as an "advance drill" for the centralized procurement of biologically similar drugs.
The above-mentioned reply from the National Medical Insurance Bureau is regarded by the industry as a signal to accelerate the centralized procurement of biologically similar drugs.
According to the principle of "more than three suppliers", "large consumption" and "large market scale", the biological analogues that can meet the requirements of centralized procurement at present (including those that are applying for registration and listing later) are mainly adalimumab, bevacizumab, rituximab, trastuzumab and infliximab.
Except that Adalimumab is used to treat rheumatoid immune diseases, the indications of the last four are basically tumor treatment. From the domestic market scale, they are all large varieties with a scale of more than 4.5 billion yuan. According to the current approval, the competition between adalimumab and bevacizumab is fierce, with 6 and 8 respectively.
Nandu Bay Financial Society noted that Adalimumab was the "king of global medicine" during the original research monopoly period. In 2021, the drug will bring Alberta a sales of 20.696 billion dollars. However, in China, in addition to five enterprises that have successfully listed the biological similar drug, more than 20 enterprises are queuing up. From the data, the biological analogues of adalimumab will increase to 4.7 billion yuan in China next year, and will reach 11.5 billion yuan in 2030.
However, bevacizumab with wide applicability is the most competitive variety. As a biological drug that has been on the market for more than 10 years, it has been "bargained" by the medical insurance, but the sales amount of bevacizumab is still further increasing. According to the data of the Southern Institute of Pharmaceutical Economics, the annual sales of the drug last year was 2.551 billion yuan, up 47.43% year on year. According to the reporter from Nandu Bay Financial News Agency, Roche, the original research party, still holds more than 50% of the market share of the drug in China. However, in the industry's view, if the centralized purchase with volume is successfully implemented, it may bring about the possibility of "overtaking at a curve" for domestic enterprises.

Biopharmaceuticals that may be included in the target of centralized procurement at present (Beibei, reporter of Cartographic Nandu Bay Finance Agency)
Although the centralized collection of biological drugs is still being promoted, if it is successfully implemented, it means that the scope of use of such drugs is widely expanded. Of course, such drugs will also face the possibility of "big price reduction".
The reporter from Nandu Bay Finance Agency learned that this year, some places actually tested the water for the collection of biologically similar drugs, and may provide some price reduction reference for the future.
In March this year, the results of 276 large varieties of alliance procurement led by Guangdong Pharmaceutical Trading Center in 11 provinces and cities were disclosed, among which rituximab was included in the alliance procurement. According to the bid winning results, Xinda Biologics was awarded the qualification of Rituximab, with a price reduction of 59%. Fuhong Hanlin and Roche were awarded the qualification of candidate, with a price reduction of 48% and 16% respectively.
It also has the corresponding reference value, which is the price reduction range of the sixth batch of national insulin procurement. According to the previous report by the reporter of Nandu Bay Financial News Agency, the average price reduction of insulin was 49% on the basis of the ceiling price. Even for the most competitive third-generation insulin, the price reduction was up to 74%.
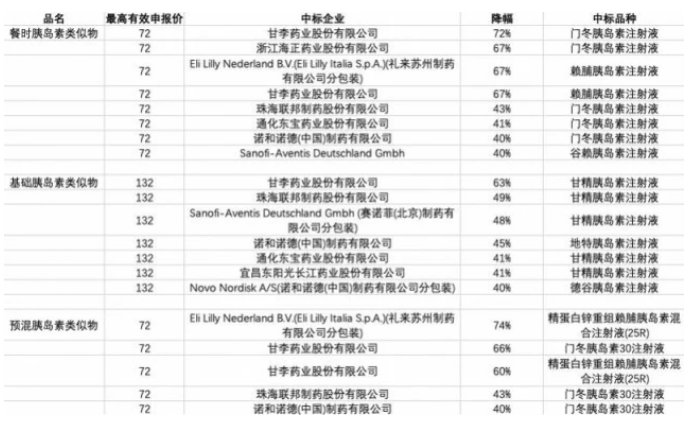
In the sixth batch of national drug procurement, although the competition for the third generation insulin was fierce, the maximum price reduction was 74% under the highest effective declared price (drawing: Beibei, reporter of Nandu Bay Finance Agency)
According to the analysis of the industry, many original biological drugs have already had corresponding biological similar drugs. The overall cost of this market is high, and the field is dominated by tumor and immune diseases, with huge expenses for patients. Therefore, the centralized procurement of this kind of drugs is "firm". However, from the perspective of the cost of drug development and preparation, the relevant departments may improve and adjust the classification, distribution and selection rules, which is similar to the possibility of "significant price reduction" of chemical drugs and "only taking the low price".
Source link: