In recent years, tumor immunotherapy has become a new method to eliminate malignant tumors. Compared with other types of immunotherapy, the new antigen vaccine, as a new type of tumor immunotherapy, can target the mutation of autologous gene, target the "target" most likely to trigger immune response, and design personalized tumor vaccine. The advantage is that targeting them can enable the patient's immune system to attack cancer cells rather than healthy cells. New tumor antigen vaccine has many forms of realization, including dendritic cells (antigen presentation), mRNA, polypeptide, DNA, etc.
•Polypeptide vaccine: after RFA, iNeo Vac-P01 was inoculated to improve patients' OS
On September 29, 2022, Newanzin Biology published a clinical study in Frontiers in Immunology. The results showed that if patients with advanced pan tumor were treated with local radiofrequency ablation (RFA) within 6 months before receiving iNeo Vac-P01 (individualized polypeptide vaccine of tumor newborn antigen), the tumor microenvironment could be further improved and the clinical efficacy could be improved.
The mechanism of peptide based tumor vaccine is that when cancer cells express antigens recognized by the host immune system and present peptides from this antigen/HLA class I complex on the cell surface, the tumor vaccine will trigger antigen specific C-cytotoxic lymphocytes to recognize peptide/HLA class I complex through T cell receptor (TCR).
In this study, 28 cancer patients were recruited, including 10 patients who received RFA treatment within 6 months before vaccination (cohort 1) and 18 patients who did not receive RFA treatment (cohort 2). Patients in cohort 1 had longer median progression free survival (mPFS) and median total survival (mOS) than patients in cohort 2 (4.42 and 20.18 months vs 2.82 and 10.94 months, respectively)
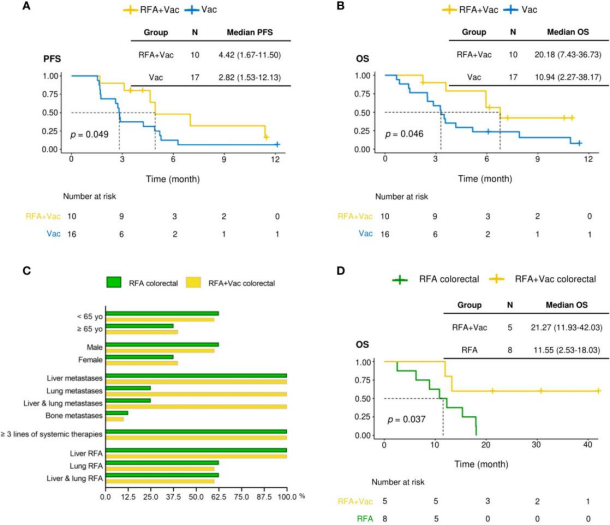
To further evaluate whether the application of iNeo Vac-P01 can improve the clinical response of patients after RFA treatment, the OS (RFA+Vac colorectal cancer) group (n=5) of colorectal cancer patients receiving RFA and iNeo Vac-P01 combined treatment was compared with the colorectal cancer patients receiving RFA (RFA colorectal cancer group) (n=8) at the same time. The mOS of RFA+Vac colorectal patients was 21.27 months (11.93-42.03 months), while that of RFA colorectal patients was 11.55 months (2.53-18.03 months), indicating that iNeo Vac-P01 vaccination after RFA can improve the OS of colorectal cancer patients.
The most common treatment-related toxicity after iNeo Vac-P01 vaccination is fatigue, fever and muscle pain. No treatment-related serious adverse events (SAE) or deaths were reported. All AEs are reversible and require no special care. This means that the combination of tumor vaccine with new antigen and RFA can safely and effectively enhance tumor inhibition.
GP2 vaccine for breast cancer, no recurrence in 5-year follow-up
In the field of peptide vaccine, there was a shocking news. At the annual meeting of breast cancer in 2020, the latest clinical trial results of GP2 vaccine were announced: all patients who received GP2 vaccine treatment had a 5-year follow-up, and the recurrence rate of breast cancer was 0%!
168 patients were included in this trial. The main purpose of this trial is to determine whether the new tumor vaccine HER2 derived peptide GP2 combined with the FDA approved immune adjuvant GM-CSF will reduce the recurrence rate of breast cancer. The study included operable HER2 positive breast cancer patients, who were randomly divided into GP2+GM-CSF and GM-CSF placebo alone after surgery.
The results showed that: after 5-year follow-up, the 5-year DFS incidence of 46 HER2+patients receiving GP2+GM-CSF treatment was 100%, without any recurrence, while the 5-year DFS (disease-free survival period) incidence of 50 placebo patients receiving GM-CSF treatment was 89.4% (95% CI: 76.2, 95.5%) (p=0.0338), and GP2 improved the 5-year survival rate by nearly 11%. Kaplan Meier survival curve shows a perfect straight line in breast cancer patients for the first time, which is equivalent to all cures!
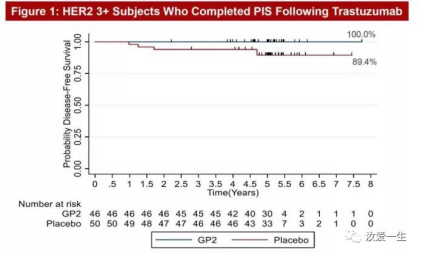
GP2 showed good tolerance without SAEs (serious adverse events), and obtained a strong immune response through local skin test and immune test, which indicated that the immune peak was reached 6 months after the completion of PIS.
NEO-PV-01 is used in combination with O medicine, with one arrow and three carves
NEO-PV-01 is a personalized new antigen vaccine, which is customized and manufactured according to the unique mutation of each patient. Its design includes up to 20 new antigen targeting peptides, aiming to generate anti-tumor immune response and guide T cells to target specific cancer new antigens in patients' tumors.
In the early clinical trial of NEO-PV-01 combined with drug O, the ORR of melanoma patients was 47%, the median PFS was not reached, and the 12 month PFS rate was 56%; The ORR of patients with non-small cell lung cancer (NSCLC) was 22%, and the median PFS was 5.6 months; The ORR of bladder cancer patients was 24%, and the median PFS was 5.6 months. This result reveals the potential of cancer vaccine as an innovative immunotherapy model.
•DC cell vaccine: Sipuleucel-T takes the lead in tumor therapeutic vaccine
DC cell vaccine Sipuleucel-T is the first tumor treatment vaccine approved by FDA, and the only prostate cancer immunotherapy made of patients' autoimmune cells. According to the latest published data, Sipuleucel-T can extend the total survival period by 14.5 months and reduce the risk of death by 45%. Become a leader in the field of tumor vaccine.
Neo MoDC and PD-1 synergize each other to eliminate the lesion for a long time
In June this year, the journal Nature published the research results of complete and lasting tumor regression in patients with advanced gastric cancer who received neo MoDC and PD-1, a new dendritic vaccine. DC cells can monitor and kill tumor cells by recognizing tumor cell specific antigens and presenting signals to T cells.
First, the doctor sequenced the whole exon of the patient's tumor tissue samples and customized her own personalized neo MoDC vaccine for new antigen dendritic cells. Initially, the personalized Neo MoDC vaccine was injected subcutaneously to start T cells. With the induction of T cell response, the tumor is still progressing. After that, doctors used Navulizumab and Neo MoDC vaccine for treatment. After 5 days, the level of CA-125 (cancer antigen 125) in the patient rapidly decreased from 596 to 64 U/ml; Two weeks later, the malignant ascites disappeared, and clinical imaging showed that the volume of metastatic supraclavicular lymph nodes decreased by about 30%; After 2 months, all metastatic lymph nodes were reduced to<1cm. The evaluation results showed that complete remission (CR) was achieved in the lymph nodes, and the length of ovarian metastatic lesions was reduced by 20%.

After 231 days, imaging examination showed that all lesions were completely resolved. On the 389th day of combined treatment, CT scan showed that ovarian metastatic cancer also completely disappeared.
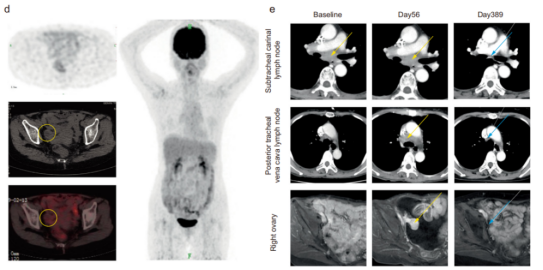
This result shows that Neo MoDC and PD-1, a new antigen vaccine, may enhance each other in combined treatment, which also provides a new immunotherapy scheme for metastatic gastric cancer and other cancers.
•DNA tumor vaccine
DNA tumor vaccine means that the encoded tumor antigen DNA is mixed with adjuvant and delivered directly to human body by intravenous injection, intramuscular injection, etc. After DC cells obtain these DNA, they need to transcribe and translate according to the sequence to present the antigen to T cells, and finally produce anti-tumor immunity.
At the annual meeting of the Society of Neurological Oncology (SNO) in 2020, Inovio announced the clinical results of two DNA drugs, INO-5401 (encoding hTERT, WT1 and PSMA) and INO-9012 (encoding IL-12), combined with Cemiplimab (PD-1 inhibitor) in the treatment of newly diagnosed glioblastoma (GBM). This new combined treatment scheme has shown good immunogenicity and tolerance in most patients.

INO-5151, a combination of INO-5150 (encoding PSA and PSMA) and NO-9012 (encoding IL-12), is being developed for the treatment of metastatic castration resistant prostate cancer (mCRPC).
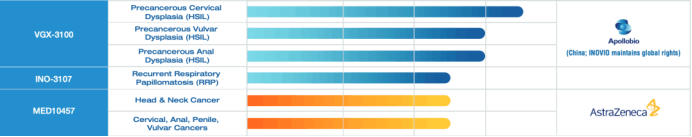
In addition, Inovio has several human papillomavirus (HPV) targeting projects, which are being developed to treat HPV related diseases. The pilot product VGX-3100 has eliminated HPV infection in nearly 50% of women with highly atypical hyperplasia and 80% of patients with highly atypical hyperplasia in the phase 2b clinical trial. At present, it has entered the phase 3 clinical trial of precancerous cervical dysplasia.
•RNA tumor vaccine
With the successful development of the novel coronavirus mRNA vaccine, it has gradually been recognized and sought after by all sectors of society, and has gradually moved towards the platform of personalized immune cell therapy, dedicated to the treatment of a variety of solid tumors.
In the cancer vaccine category, Moderna has two noteworthy individualized cancer vaccines. The leading product, mRNA-4157, can encode up to 34 new antigens. In the first phase of clinical trial, patients with head and neck squamous cell carcinoma (HNSCC) were treated with pabolizumab. The total remission rate (ORR) reached 50%, and the median progression free survival period (mPFS) was 9.8 months compared with the previous data of pabolizumab single drug treatment: ORR 14.6%, mPFS 2.0 months), showing huge therapeutic potential. The second phase of clinical trial is in progress. MRNA-5671 is a personalized tumor vaccine targeting KRAS mutation. It has entered the current phase 1 clinical trial and is carried out in a variety of solid tumor patients with KRAS mutation.
CureVac's leading product, CV8102, is a TLR7/8 and RIG-1 agonist based on non coding single strand RNA. Although there are cancer vaccines targeting tumor related antigens and newborn antigens in its clinical pipeline, they are still in the pre clinical development stage, and no specific information has been disclosed.
•Phase III clinical trial of new epitope vaccine Tedopi was successful
Tedopi (OSE-2101) is also a cancer vaccine. Its special feature is that it is a new epitope vaccine. It uses targeting methods to combat tumor heterogeneity by selecting well expressed antigen determinants found in a variety of tumor types.
In the previous phase III Atlante-1 clinical study, patients with advanced NSCLC were included and randomly divided into Tedopi group or chemotherapy group (pemetrexed or docetaxel) according to 2:1. The results showed that among 63 patients in the Tedopi group, 29 patients survived at least 12 months, and the 12 month survival rate was 46%, higher than the predetermined 25%. The observed 46% survival rate is also higher than the 40% survival rate of the alternative effect assumption in the protocol. Compared with the chemotherapy group, 13 of the 36 patients survived at least 12 months, equivalent to a 12 month survival rate of 36%.
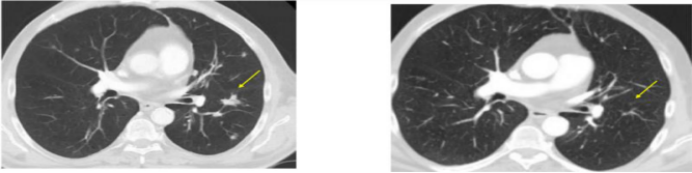
And in a real treatment case, after 5 times of vaccine injection, the tumor of patients with advanced lung cancer shrank rapidly (from 39 mm to 23 mm), and the progression free survival period was 4.2 months.
Summary:
With the in-depth exploration of the interaction mechanism between tumor and immune system, the new tumor antigen vaccine will be the prelude to precise treatment. Although the road of research and development and application is extremely difficult, with the continuous deepening of research on new antigens and the positive feedback obtained after the continuous trial of DC cells, DNA, RNA and other vaccines, it is believed that in the near future, the good news of precise immunization will be delivered to tumor patients.
Article Source:
https://www.medsci.cn/article/show_article.do?id=d8c7e4394932