Colorectal cancer is a common malignant tumor. According to the previous survey data in China, about 83% of colorectal cancer patients have developed to the advanced stage when they are diagnosed, and drug treatment is very important. In recent years, targeted therapy has become an important method for the treatment of malignant tumors with an overwhelming momentum. There are a wide variety of targeted drugs. Up to now, FDA has approved a total of 11 precision treatment drugs, including 8 targeted drugs (EGFR, VEGF, BRAF, etc.) and 3 immunotherapeutic drugs. So how to choose these drugs? Xiaobian will show you how to sort it out.
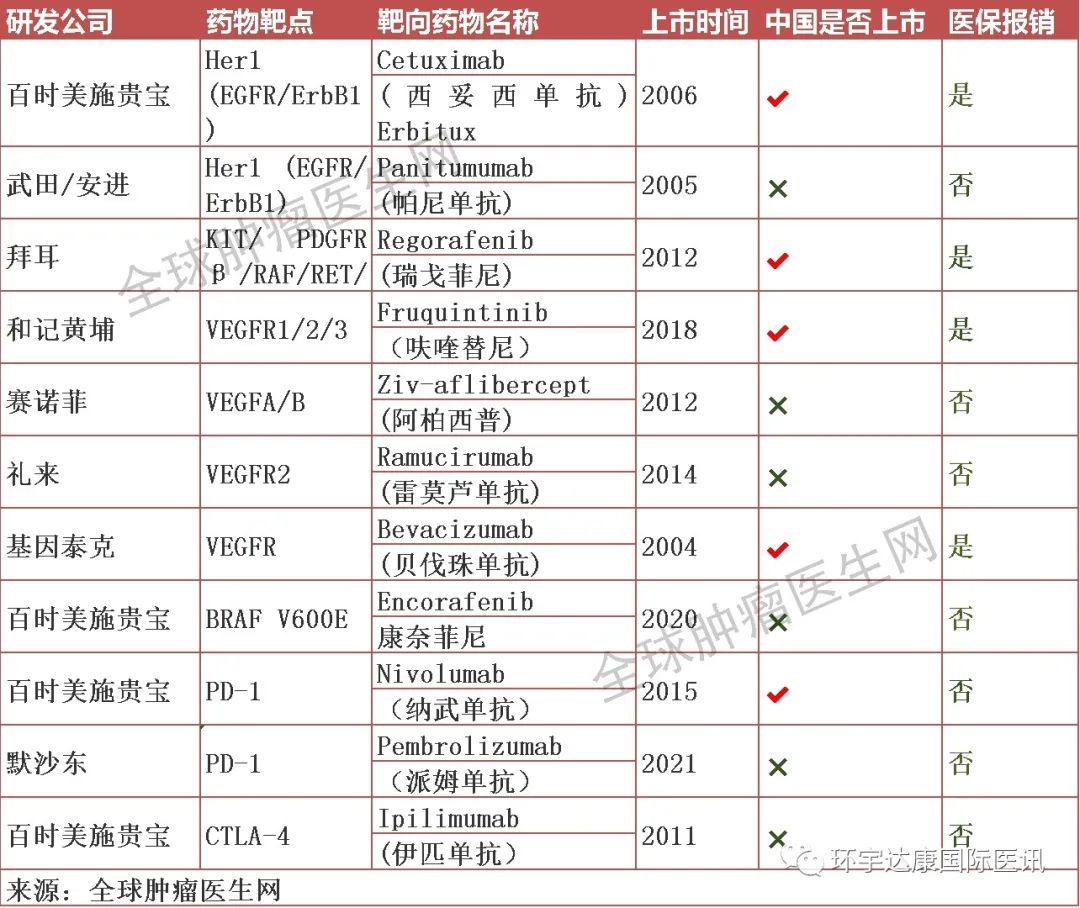
Names and targets of targeted drugs for colorectal cancer
1、 EGFR
Epidermal growth factor receptor (EGFR) occurs in about 10% of colon cancers, most commonly on the left.
1. Cetuximab Erbitux
Target: EGFR
indication:
Cetuximab and irinotecan can be used in combination with irinotecan in patients with advanced colorectal cancer who have poor efficacy with irinotecan alone or in combination with other chemotherapy drugs;
Cetuximab can be used alone in patients who cannot tolerate irinotecan.
Drug information: the US Food and Drug Administration (FDA) first approved Erbitux (cetuximab) in 2004 to treat patients with advanced colorectal cancer expressing EGFR who failed other treatments; In the 2009 NCCN colorectal cancer guidelines, it is suggested that cetuximab combined with chemotherapy can be selected as the first-line treatment for patients with RAS wild-type advanced metastatic colorectal cancer.
2. Panitumumab vectibix
Target: EGFR
indication:
Colorectal cancer with EGFR overexpression whose condition is still progressive or metastatic after chemotherapy with fluorouracil, oxaliplatin and irinotecan;
Combined FOLFOX regimen was used for the first-line treatment of KRAS wild-type MCRC patients.
Drug information: in 2006, FDA approved vectibix (panitumumab) single drug treatment for metastatic colorectal cancer expressing EGFR, previous chemotherapy regimen failure of fluoropyrimidine, oxaliplatin and irinotecan. In 2014, FDA expanded the indications and approved vectibix combined with FOLFOX for the first-line treatment of metastatic colorectal cancer expressing wild-type KRAS (exon 2); In 2017, the US FDA expanded its indications again and approved vectibix to treat metastatic colorectal cancer expressing wild-type ras (both KRAS and NRAS express wild-type).
2、 VEGF
The proliferation of tumor tissue requires the formation of a large number of new blood vessels around it to transport oxygen and nutrition. This process is realized by tumor through human vascular endothelial growth factor (EGFR). Anti angiogenesis drugs inhibit the activity of human vascular endothelial growth factor, and then inhibit tumor proliferation.
3. Bevacizumab, Avastin
Target: VEGF
Indications: Bevacizumab combined with FOLFIRI regimen as the first-line treatment for advanced colorectal cancer.
Drug details: approved by FDA on february26,2004, Avastin ®, Roche Pharma) is the first drug approved for marketing in the United States to inhibit tumor angiogenesis. In 2006, US FDA approved bevacizumab combined with FOLFOX4 (5-fluorouracil, calcium folinate and oxaliplatin) for the first-line treatment of metastatic colon or rectal cancer. Bevacizumab is a recombinant humanized monoclonal antibody against vascular endothelial growth factor (VEGF), which can bind to VEGF and prevent angiogenesis. Several trials have shown that bevacizumab can significantly improve the quality of life and improve the progression free survival (PFS) of patients. It should be noted that bevacizumab is generally more effective in patients with tumors with high expression of EGFR.
4. Cyramza, ramucirumab
Target: VEGFR2
Indications: combined with FOLFIRI regimen, it is used to treat patients with metastatic colorectal cancer who have disease progression during or after treatment with bevacizumab, oxaliplatin and fluorouracil.
Drug details: on april24,2015, FDA approved the combination of ramoruzumab and FOLFIRI regimen for the treatment of patients with metastatic colorectal cancer who had disease progression during or after the treatment with bevacizumab, oxaliplatin and fluorouracil. Cyramza is a monoclonal antibody drug, which mainly binds to vascular endothelial growth factor receptor (VEGFR2). As the tumor tissue grows, it will go through the process of angiogenesis, that is, to form new blood vessels around the tumor tissue to transport nutrients to the tumor cells. So cyramza is an optional drug for most patients with lung cancer. Therefore, inhibition of this process can inhibit the proliferation of most tumors. See the following figure:
5. Regorafenib
Action target: multiple targets
Indications: Patients with metastatic colorectal cancer (mCRC) who have previously received chemotherapy based on fluorouracil, oxaliplatin and irinotecan, and who have previously received or are not suitable for anti VEGF treatment and anti EGFR treatment (RAS wild type)
Drug information: regafini is a new type of multi kinase inhibitor, which blocks a variety of enzymes that promote tumor growth. It inhibits angiogenesis around the tumor by inhibiting the activity of vegfr2-tie2, which inhibits the nutrient supply of the tumor, thus reducing the tumor and controlling the tumor progression. In 2012, FDA approved the oral drug regorafenib for the treatment of metastatic colorectal cancer. The specific indications are metastatic colorectal cancer (CRC) previously treated with chemotherapy based on fluoropyrimidine, oxaliplatin and irinotecan, anti VEGF treatment and anti EGFR treatment (if KRAS wild type). In may2017, China's CFDA also approved regofinil to be listed.
6. Zaltrap Ziv Aflibercept
Target: vegfa/b
Indications: it is used together with FOLFIRI to treat colorectal cancer that has metastasized (spread to other parts of the body). It is used for patients whose condition has not improved after other chemotherapy.
Drug information: in 2012, abecept was approved by FDA to treat advanced colorectal cancer. It is a chimeric protein drug, which restricts tumor nutrition supply by inhibiting human vascular endothelial growth factor VEGF, and then inhibits tumor proliferation.
7. Fruquintinib
Target: vegfr1/2/3
Indications: for patients with metastatic colorectal cancer who have previously received chemotherapy based on fluorouracil, oxaliplatin and irinotecan, and who have previously received or are not suitable to receive anti vascular endothelial growth factor (VEGF) treatment and anti epidermal growth factor receptor (EGFR) treatment (RAS wild type).
Drug information: in 2020, the US Food and Drug Administration (FDA) has awarded fruquintinib as a fast track for the treatment of patients with metastatic colorectal cancer (mCRC). These patients had previously received chemotherapy based on fluoropyrimidine, oxaliplatin and irinotecan, as well as anti vascular endothelial growth factor (VEGF) biotherapy.
3、 BRAF V600E
7-10% of colon cancer patients carry BRAF V600E mutation. BRAF V600E mutation belongs to BRAF activation mutation, which is the variant form with the highest proportion of BRAF. Unique clinical features:
It mainly appeared in the right colon;
The proportion of dmmr is high, reaching 20%;
The prognosis of BRAF V600E mutation was poor;
Atypical transfer mode;
Patients with BRAF mutations usually have a poor prognosis, and some new precision anticancer drugs have been shown to double the survival time.
8. Encorafenib
Target: BRAF V600E
Indications: Patients with metastatic colorectal cancer (mCRC) carrying BRAF V600E mutation. These patients have already received one or two previous therapies.
Drug information: on April 8th, 2020, FDA approved braftovi ® (encorafenib, Cornelius) and Erbitux ® Cetuximab is the first FDA approved targeted therapy for patients with MCRC carrying BRAF gene mutation.
4、 Pd-1/l1
9. Keytruda (pembrolizumab)
Target: PD-1
Indications: cancer in patients with unresectable or metastatic microsatellite instability (MSI-H) or mismatch repair defect (dmmr) colorectal cancer
Drug details: on june29,2020, the US Food and Drug Administration (FDA) approved the use of keytruda (merck&co) in the first-line treatment of patients with unresectable or metastatic colorectal cancer with high MSI-H or mismatch repair defect (dmmr).
10. Opdivo, nivolumab
Target: PD-1
Indications: Patients with microsatellite instability (MSI-H) or mismatch repair defect (dmmr) after platinum chemotherapy in adults or children (≥ 12 years old) with metastatic colorectal cancer (mCRC).
Drug details: in August, 2017, FDA accelerated the approval of intravenous infusion of nivolumab for patients with high microsatellite instability (MSI-H) or mismatch repair defect (dmmr) in adults or children (≥ 12 years old) with metastatic colorectal cancer (mCRC) after treatment with fluorouracil, oxaliplatin and irinotecan.
5、 CTLA-4
11、Ipilimumab +nivolumab
Target: PD-L1
Indications: ipilimumab (ipilimumab) combined with nivolumab (navumab) for patients with metastatic colorectal cancer (mCRC) who have progressed after treatment with fluorouracil, oxaliplatin and irinotecan
Drug details: on july10,2018, FDA accelerated the approval of ipilimumab (ipimab) and nivolumab (navumab) in combination for the treatment of metastatic colorectal cancer (mCRC) patients aged 12 years and over, with high microsatellite instability (MSI-H) or mismatch repair defect (dmmr), and after treatment with fluorouracil, oxaliplatin and irinotecan.
Recent advances in targeted therapy for colorectal cancer
In addition to the above targeted therapies approved by the US FDA, the researchers also found that the three drug combination scheme of braftovi (encorafenib) +erbitux (cetuximab) +mek inhibitor mektovi (binimetinib) has a significant effect on the treatment of metastatic colorectal cancer, and significantly prolongs the survival time compared with the scheme of irinotecan combined with cetuximab.
In addition, new breakthroughs have been made in the treatment of KRAS mutations in colorectal cancer. Lumakras (sotorasib), the world's first KRAS targeted drug, also has significant effects in the field of colorectal cancer treatment. The results of phase I codebreak 100 trial showed that the disease control rate of patients with KRAS G12C mutant colorectal cancer treated by lumakras (sotorasib) reached 76.2%, and the vast majority of patients had good tolerance to the treatment.
Article source: