Lung cancer can be roughly divided into two histological subtypes: non-small cell lung cancer (NSCLC) and small cell lung cancer (SCLC). These two major subtypes can be further divided into different subtypes according to their different characteristics. Now we know that many non-small cell lung cancers are caused by driver gene mutations, and this type of lung cancer is suitable for targeted treatment. In addition, immunotherapy is more and more widely used in the treatment of lung cancer.
There has been a lot of progress in screening, early detection, diagnosis and treatment of lung cancer, especially in selecting individualized treatment according to biomarkers (driver gene mutation, PD-L1 expression, tumor mutation load, etc.). Lung cancer is the solid tumor with the most clinical trials. Let's take a look at the global development of clinical drugs for lung cancer in 2021.
Which new drugs for lung cancer have been developed most?
Among 707 new drugs under development,
1 there are 590 kinds of non-small cell lung cancer (NSCLC):
There are 214 new immunooncology drugs, of which 57% are in phase II or III clinical trials;
There are 376 new non immune oncology drugs, of which 55% are in phase II or III clinical trials. Among these drugs, the vast majority are targeted therapeutics, 309 of which are prolific signaling inhibitors. There are 166 compounds, 87 of which are in phase II and III clinical trials.
2 there are 117 kinds of small cell lung cancer (SCLC):
36 new immunooncology drugs, including 15 immune checkpoint inhibitor therapies. 50% of new drugs are in phase II and III clinical trials;
81 new non immune oncology drugs. Targeted therapy is still the largest category, with 54 kinds, of which 43 are in phase II and III clinical trials.
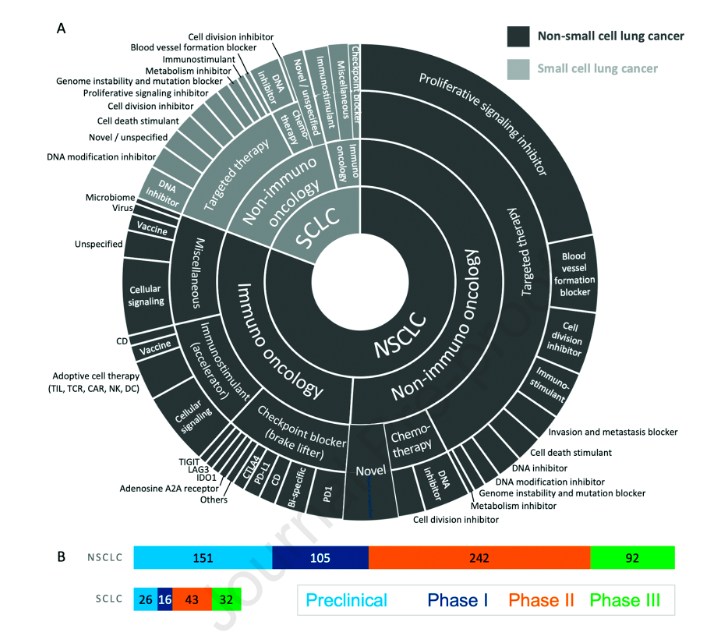
Figure 1. Classification of new drug R & D for lung cancer
Which countries / regions have the most research and development of new drugs?
In terms of the number of research and development of new drugs for lung cancer treatment, the United States dominates the world. Of the 590 new drugs for the treatment of non-small cell lung cancer, 246 have been developed in the United States. Among the 117 new drugs for small cell lung cancer, the United States has developed 55.
The second largest R & D country is China, which has developed 123 new drugs, including 107 new drugs for non-small cell lung cancer and 16 new drugs for small cell lung cancer.
New immunooncology drugs for small cell lung cancer have only been developed in six countries and regions, including the United States, China, Switzerland, Australia, the United Kingdom and Hong Kong.
The research and development scope of new non immune oncology drugs for non-small cell lung cancer is much larger, with 376 new drug developments covering 26 countries and regions in the world.

Figure 2. Number of new drug R & D in different countries and regions
The research and development of new drugs for targeted therapy is dominant
Among the new drugs in non immune oncology of non-small cell lung cancer, targeted therapy still dominates, 309 of 376 new drugs, most of which are tyrosine kinase inhibitors (TKIs) that target cell proliferation signal pathway genes, such as ALK, BRAF, EGFR / HER2 / erbB2 family, KRAS, met, ntrk, RET and ros1.
For tumors with EGFR and alk mutations undergoing multi-line treatment, the development of these new drugs is expected to solve the problem of acquired drug resistance of existing target drugs. In addition, for less common driver genes, such as RET, ros1 and ntrk, patients carrying these mutations have fewer treatment options, and new treatment options are expected to appear in these new drugs.
In addition to tyrosine kinase inhibitors, many small molecule inhibitors targeting genomic instability and tumor metabolism are also being studied, including histone deacetylases (HDAC1 and HDAC6), Hsp90, MDM2 / 4, PARP / tankyrase, pegagiminase, glutaminase and fatty acid synthase. These new drug R & D have brought new hope to those patients who have progressed and are looking for other options after using multi line TKIs.
Similar to non-small cell lung cancer, targeted therapies account for the vast majority of new drug development in small cell lung cancer. The treatment options for small cell lung cancer are limited, and traditional chemotherapy is the main one, because the frequency of driver gene mutations in small cell lung cancer is very low. The development of targeted drugs for small cell lung cancer focuses on drugs targeting histone demethylases and deacetylases, Brd4, ezh1 / 2 and MDM2 / 4.
Immunotherapy research and development continues in full swing
There are many trials in the field of lung cancer for the development of new immunotherapy or the combination of immunotherapy and other therapies.
Among 214 new immune drugs for non-small cell lung cancer:
70 are immune checkpoint inhibitors we often hear about (targeting PD-1, PD-L1, CTLA-4), as well as bispecific drugs (such as pd-l1/tbf) combined with them and other drugs β 1);
72 are immunostimulators of innate and adaptive immune responses, mainly targeting key cellular signaling pathways, such as angiogenesis, cancer-related microbiota, macrophages that inhibit immunity, etc. Oncolytic viruses and cancer vaccines also fall into this category. In addition, there are many therapies based on immune cells, such as car T-cell therapy.
There are also many immunotherapy developments in the field of small cell lung cancer. Among the 36 new drugs, 15 are immune checkpoint inhibitors and 12 are immune stimulants. Similarly, PD1, PD-L1, and CTLA4 are the main targets of immune checkpoint inhibitors. However, LXR, CD47, tigit and Lag3 inhibitors have also received a lot of attention. Some new targets of immunotherapy for small cell lung cancer include CSF2, antxr, cadherin and macrophages.
Summary
It is believed that the research and development of these new drugs in the field of lung cancer can bring more and better treatment options and long-term clinical benefits to patients. Researchers are making continuous efforts. We should remain optimistic and look forward to the progress of medicine bringing more hope to patients!
Source link:
https://www.cn-healthcare.com/articlewm/20220906/content-1430905.html