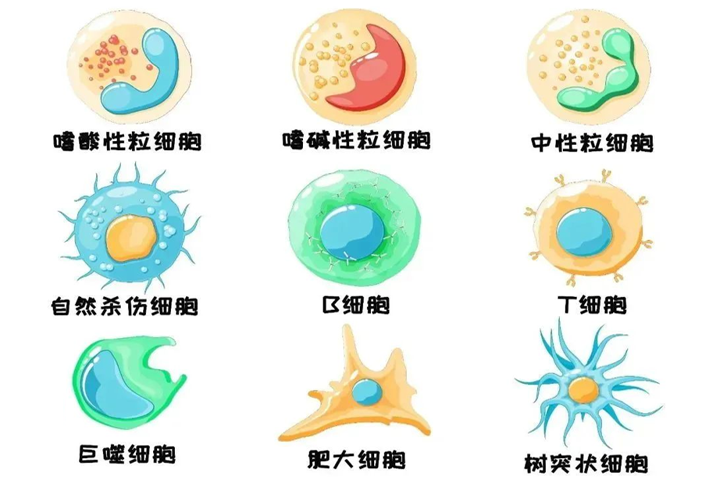
In the past ten years, from initial efficacy to successful marketing, PD-1/PD-L1 immunotherapy is one of the most successful tumor immunotherapies, which has successfully set off a revolution in tumor treatment and led the transformation of cancer treatment!
Cancer immunotherapy fights against cancer by releasing the ability of the patient's immune system. Although tumor immunotherapy has developed late, it has entered clinical practice at an unprecedented rate. In recent years, it has become the fifth pillar of cancer treatment and is expected to eventually become an important weapon to cure tumors. In 2022, FDA approved 10 new immunotherapies, including TCR-T, the world's first solid tumor cell immunotherapy, relatlimab, the world's first LAG3 inhibitor, and so on, bringing more choices and hope to all kinds of cancer patients!
1、 The world's first solid tumor TCR-T cell immunotherapy was approved
On January 25, 2022, FDA approved tebentafusp tebn (Kimmtrak, Immunocore Limited), a double specific gp100 peptide HLA directed CD3 T cell conjugator, for use in HLA-A * 02:01 positive, unresectable or metastatic uveal melanoma. It is worth mentioning that this is the world's first anti-cancer therapy approved by FDA in 2022, and also the world's first approved TCR-T therapy for solid tumors, which means that T cell therapy officially challenges solid tumors, with milestone significance! Original link: Milestone! The world's first TCR-T therapy for solid tumors has been approved for marketing!
2、 The first domestic CAR-T therapy was approved for myeloma
On February 28, 2022, FDA approved Carvykti, a legendary BCMA CAR-T targeted therapy, to be marketed for the treatment of patients with relapsed or refractory multiple myeloma (R/R MM). These patients had received four or more treatments in the past, including proteasome inhibitors, immunomodulators and anti CD38 monoclonal antibodies, which brought new choices to patients who had no way out in the late stage, At the same time, it also has its own name -- Sidakiolence. It is worth mentioning that this is the first domestic CAR-T immunotherapy that has been approved by FDA for listing, which means that China's CAR-T immunotherapy has officially entered the world stage! This is another milestone in China's anti-cancer history worth carving! Original link: Milestone! FDA approved the launch of Carvykti, the first domestic CAR-T therapy! The price and indications have been announced
3、 The first neoadjuvant therapy for non-small cell lung cancer - OPDIVO+chemotherapy scheme approved
On March 4, 2022, the US FDA approved Opdivo (nivolumab, drug O) combination chemotherapy as a new adjuvant therapy to treat resectable (tumor ≥ 4cm or lymph node positive) non-small cell lung cancer patients, regardless of the patient's PD-L1 expression. This is the first and only approved NSCLC preoperative new adjuvant treatment program. Related reading: Version 2022 CSCO Guidelines for Non Small Cell Lung Cancer is released!
4、 The world's first LAG-3 immunotherapy has been approved for marketing! The birth of "the strongest" double immunotherapy in history
On March 18, 2022, FDA approved the fixed dose combination of relatlimab and nivolumab for the treatment of adults and children aged 12 years or above with unresectable or metastatic melanoma. This "golden partner" also has its own name - Opduraag. This is the first LAG-3 inhibitor in the world, and also the third immune checkpoint for clinical application after PD-1 and CTLA-4, with milestone significance! This new immunotherapy with better effect will bring new "cure" hope to cancer patients!
5、 Endometrial cancer ushers in the 30th immunotherapy
On March 22, 2022, FDA approved the new indication of Pembrolizumab (Keytruda) for advanced endometrial cancer patients with high microsatellite instability (MSI-H) or mismatch repair defect (dMMR) after frontline systemic treatment. These patients must not be suitable for curative surgery or radiotherapy. The good news is that in addition to pemtuzumab, many new drugs for endometrial cancer are currently in clinical recruitment, and patients who want to participate can leave a message.
6、 The world's first second-line CAR-T therapy for large B-cell lymphoma
On April 1, 2022, FDA approved the CAR-T cell therapeutic drug Yescarta for adult patients with large B-cell lymphoma (LBCL) who are refractory to first-line chemotherapy immunotherapy or relapse within 12 months after first-line chemotherapy immunotherapy. This is also the world's first CAR-T drug approved by FDA as a second-line therapy for LBCL.
7、 Immune combination therapy approved for first-line treatment of esophageal cancer
On May 27, 2022, the US FDA approved two combined treatment schemes of immunotherapy with Navulizumab (Opdivo, i.e. drug O) (drug O is used in combination with fluoropyrimidine and platinum containing chemotherapy; drug O is used in combination with aspirin (drug Y)) as first-line therapy to treat advanced unresectable or metastatic esophageal squamous cell carcinoma (ESCC), regardless of the patient's PD-L1 status. These two combination therapies based on Navulizumab have shown great survival benefits, providing better treatment options for patients with esophageal cancer.
8、 First follicular lymphoma CAR-T therapy approved
On May 27, 2022, the US FDA accelerated the approval of tisagenlecleucel (Kymarih) for the treatment of adult patients with recurrent or refractory follicular lymphoma (FL) after two or more lines of systemic treatment. This is the third indication of Kymarih approved by FDA, and the only approved CAR-T cell therapy among adult/child applicable therapies. The good news is that many CAR-T therapies are currently in clinical recruitment, and a large number of patients have successfully received CAR-T therapy through the global doctor network.
9、 Second second-line CAR-T therapy for large B-cell lymphoma approved
On June 25, 2022, FDA approved Breyanzi (lisocabtagene maraleucel, liso cel) for the second-line treatment of adult large B-cell lymphoma patients (including diffuse large B-cell lymphoma, high-grade B-cell lymphoma, primary mediastinal large B-cell lymphoma and follicular lymphoma grade 3B).
10、 The first immunotherapy for cholangiocarcinoma was approved
On September 2, 2022, FDA approved the supplementary biological agent license of devaruzumab (Durvalumab, Imfinzi), which is used in combination with gemcitabine and cisplatin to treat patients with locally advanced or metastatic cholangiocarcinoma. It is worth mentioning that this is the first immunocheckpoint inhibitor therapy for patients with cholangiocarcinoma. Clinical data show that the use of PD-L1 inhibitor devarumab in combination with gemcitabine and cisplatin can significantly improve the overall survival rate of patients compared with chemotherapy alone.
In the future, cancer cure will eventually become a reality!
Cancer research is in an exciting era. In recent years, the discovery of cancer genomics and immunology has successfully established two pillars of cancer treatment - targeted therapy and immunotherapy. They benefit more patients with different types of cancer.
With the development of these new anti-cancer drugs, vaccines and monoclonal antibodies, the terminal cancer, which is called "terminal disease", will become a chronic disease, fundamentally changing the concept of cancer treatment. We hope that 20 years from now, mankind will be able to vaccinate against cancer and defeat it. I believe that this day is not far away!
Source:
https://www.cn-healthcare.com/articlewm/20221019/content-1452653.html