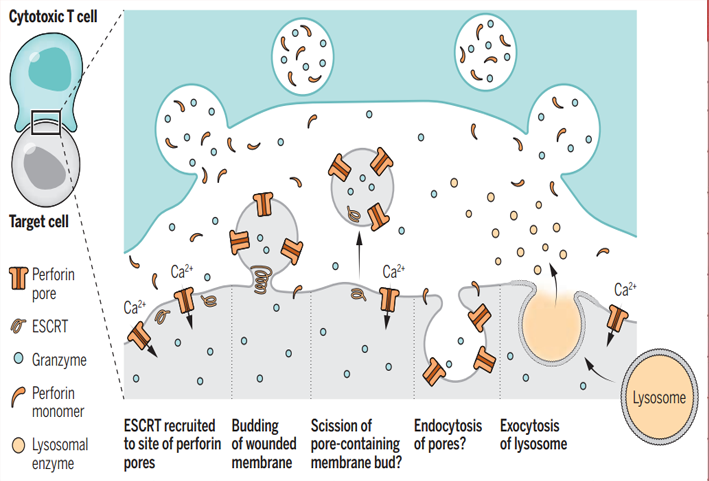
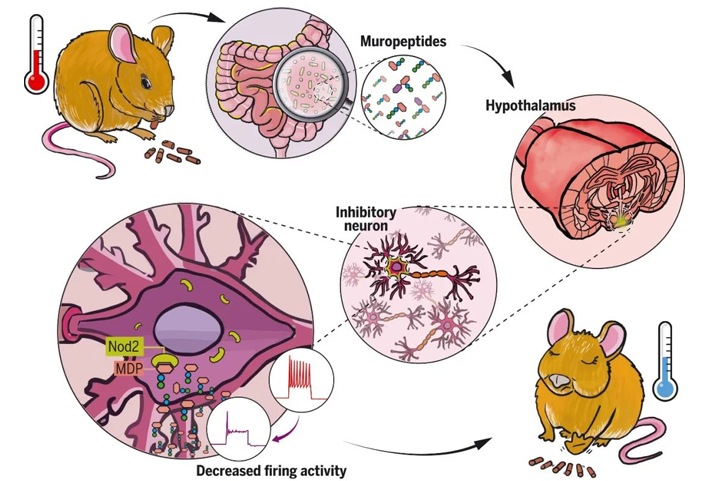
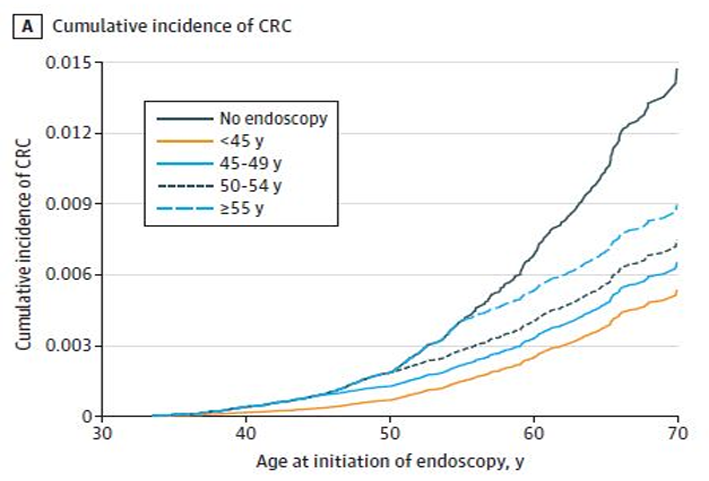
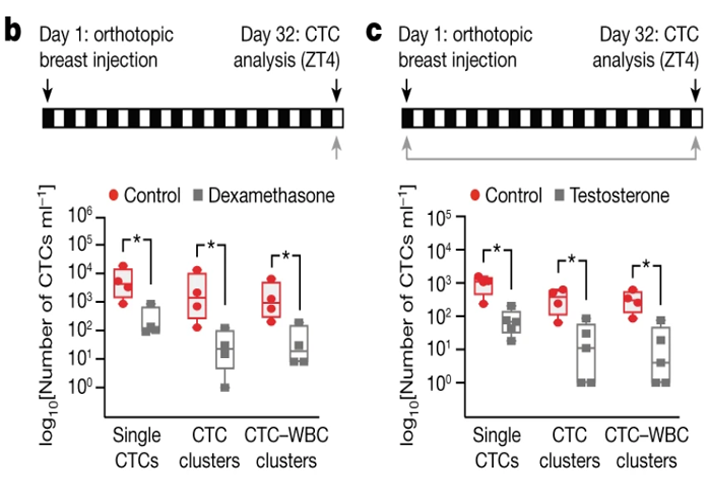
CNR Shanghai on November 30, Junshi biology announced that the new indication of treprizumab injection (tuoyi), an anti-PD-1 monoclonal antibody independently developed by the company, combined with cisplatin and gemcitabine for first-line treatment of patients with locally recurrent or metastatic nasopharyngeal carcinoma, has been approved by the State Drug Administration.
This is the second indication of treprizumab in the field of nasopharyngeal carcinoma. In February this year, treprizumab was approved for the treatment of patients with recurrent / metastatic nasopharyngeal carcinoma (NPC) who had failed to receive second-line and above systematic treatment, becoming the first anti-PD-1 monoclonal antibody approved for the treatment of NPC in the world. The "next city" of treprizumab in the first-line treatment of nasopharyngeal carcinoma will bring innovative treatment schemes and longer survival benefits to patients at different treatment stages.
Nasopharyngeal carcinoma (NPC) is one of the most common head and neck tumors, which is a kind of primary malignant tumor of nasopharyngeal mucosa. According to statistics, in 2020, the number of new cases of nasopharyngeal carcinoma in the world exceeded 130000, of which nearly half were in China. For recurrent or metastatic nasopharyngeal carcinoma, the current treatment methods are limited. The first-line standard treatment is platinum based two drug combined chemotherapy, but the median progression free survival time after treatment is only about 7 months.
Xu Ruihua, Professor of cancer prevention and treatment center of Zhongshan University, has worked with local innovative pharmaceutical company Jun Shi biology. Since 2016, we have explored innovative clinical programs combining new immunotherapy with traditional chemotherapy. From the small sample size exploratory study of phase Ib / II, the polaris-02 study of phase II single drug second-line and back-line immunotherapy, to the jupiter-02 study of the first-line treatment of "immunotherapy + chemotherapy" in the world's largest international multi center, treprizumab has obtained solid evidence-based medical evidence in the field of nasopharyngeal carcinoma treatment.
Source: cnr.com
http://www.cnr.cn/shanghai/qqlb/20211130/t20211130_525675271.shtml