Tissue and biomarker analysis
1. is it necessary to accurately identify all EGFR mutations with clinical utility covering common and atypical / rare mutations?
Consensus: it is suggested that it is best to use second-generation sequencing to extensively detect exons 18-21 of EGFR tyrosine kinase region in order to find all mutations with clear or potential clinical utility. (evidence level: I, recommendation level: a)
2. What is the role of tissue re biopsy in NSCLC patients with EGFR mutations after receiving TKI treatment progression?
Consensus: after receiving TKI treatment progression, NSCLC patients with EGFR mutations are recommended to undergo tissue biopsy (if feasible) to evaluate the targetable drug resistance mechanism and potential histological transformation. (evidence level: I, recommendation level: a)
3. what is the role of cfdna detection before and after the use of EGFR TKIs?
Consensus: if no tissue is available, cfdna testing is valuable to help identify EGFR mutations and some drug resistance mechanisms after initial diagnosis and drug resistance. (evidence level: I, recommendation level: a)
4. Should we detect EGFR and PD-L1 simultaneously or sequentially?
Consensus: PD-L1 testing should be performed concurrently with EGFR (and other biomarkers) to allow rapid classification in the absence of targetable gene alterations. (evidence level: I, recommendation level: a)
5. is it necessary to extend the detection recommendations for advanced EGFR mutations to those patients with non squamous NSCLC who have undergone radical resection?
Consensus: Yes, postoperative adjuvant targeted therapy in patients with non squamous NSCLC requires EGFR mutation detection. (evidence level: I, recommendation level: a)
6. what is the biological background and therapeutic relevance of EGFR 20 exon insertion mutations?
Consensus: EGFR 20 exon insertion mutations can activate EGFR tyrosine kinase signaling and have therapeutic relevance because specific targeted therapies against this group of mutations are emerging. (evidence level: I, recommendation level: a)
7. is it necessary to detect and report co mutations that occur with EGFR mutations in advanced NSCLC?
Consensus: finding co mutations with EGFR mutations in advanced NSCLC may be a poor prognostic indicator and may predict relative resistance to EGFR TKIs. In the absence of direct therapeutic significance, a study investigation of coexisting mutations can be carried out, but is not necessary. (evidence level: I, recommendation level: a)







Early and locally late
1. What is the role of ositinib in the adjuvant treatment of patients with EGFR sensitive mutations and stage ib-iiia NSCLC after surgery?
Consensus: to date, ositinib has been recommended for three years as an adjuvant therapy for patients with resected stage ib-iiia (7th TNM) NSCLC carrying EGFR mutations. So far, ositinib has been recommended as an adjuvant therapy for patients with EGFR sensitive mutations and stage ib-iiia NSCLC for three years. Significant improvements in disease-free survival (DFS), including better central nervous system control, should ideally be supported by overall survival and / or quality of life benefits after mature follow-up. (evidence level: I, recommendation level: a)
2. What is the status of the first or second generation EGFR-TKIs for the postoperative adjuvant treatment of patients with EGFR sensitive mutations and stage ii-iiia NSCLC?
Consensus: there is no conclusive evidence to suggest the use of first - or second-generation EGFR TKIs as adjunctive therapy for surgically resected EGFR mutant NSCLC. (evidence level: I, recommendation level: a)
3. How to follow up after complete resection of EGFR mutated NSCLC?
Consensus: patients should be followed up according to the current ESMO guidelines for early NSCLC. Considering the high risk of CNS metastasis in NSCLC patients with EGFR mutations, brain imaging should be performed every 6 months during follow-up (MRI is preferred). (evidence level: I, recommendation level: a)
4. Should patients with surgically resected stage IB IIIa and EGFR mutated NSCLC receive adjuvant chemotherapy?
Consensus: adjuvant chemotherapy is strongly recommended for NSCLC patients with surgically resected stage IB IIIA (7th TNM), EGFR mutation and good physical condition, regardless of whether targeted therapy is used. For high-risk stage IB patients (7th TNM) with negative margin, adjuvant chemotherapy may be considered if they are in good physical condition. (evidence level: I, recommendation level: a)
5. What is the role of adjuvant ositinib in patients with EGFR mutations and early resection of NSCLC who do not meet the inclusion criteria of adaura?
Consensus: Although there is no data specifically for this population, it can be inferred from adaura data that adjuvant treatment with ositinib can be considered for patients with incomplete resection (less than lobectomy) or residual lesions, or EGFR mutated NSCLC after radiotherapy. For patients with rare EGFR mutations, treatment needs to be individualized. (evidence level: II, recommendation level: C)
6. For patients with EGFR mutated NSCLC who meet the inclusion criteria of the adaura study but have clinical concerns about the tolerance of ositinib, what is the role of postoperative adjuvant therapy with ositinib?
Consensus: patients who may be eligible for adjuvant ositinib treatment according to EGFR genotype, surgical resection and pathological stage (adaura inclusion criteria), but have clinical tolerance problems, such as old age, interstitial pneumonia and other comorbidities, incomplete postoperative recovery, ECoG PS score ≥ 2, heart damage or history of malignant tumor, may consider adjuvant ositinib treatment according to specific circumstances. Every effort should be made to optimize comorbidities and conduct additional safety monitoring, taking into account that the individualized risk of adverse events is balanced with potential disease-free survival time benefits and unknown overall survival effects. (evidence level: II, recommendation level: C)
7. what is the role of EGFR TKIs for early EGFR mutated NSCLC patients who receive stereotactic radiotherapy (SBRT) instead of surgery?
Consensus: in NSCLC patients with early EGFR mutations, simultaneous or sequential treatment of EGFR TKI and SBRT is currently not recommended. (evidence level: III, recommendation level: C)
8. What is the role of neoadjuvant EGFR TKI in patients with stage ia-iiia NSCLC who are operable or borderline operable (e.g., T3 / T4)?
Consensus: there is currently no data to support the use of neoadjuvant EGFR TKI therapy in patients with operable or borderline operable NSCLC. (evidence level: II, recommendation level: C)
9. What is the role of consolidation immune checkpoint inhibitor therapy in patients with inoperable EGFR mutated stage III NSCLC after receiving radical chemoradiotherapy?
Consensus: in EGFR positive diseases, the use of consolidation immune checkpoint inhibitors after radical chemoradiotherapy (CT-RT) is not recommended. (evidence level: I, recommendation level: C)
10. What is the role of TKI before, after and during radiotherapy in stage III EGFR mutant NSCLC patients who cannot be operated?
Consensus: Currently, it is not recommended to use stage III EGFR mutated NSCLC patients who cannot be operated before, during or after radiotherapy. (evidence level: III, recommendation level: C)
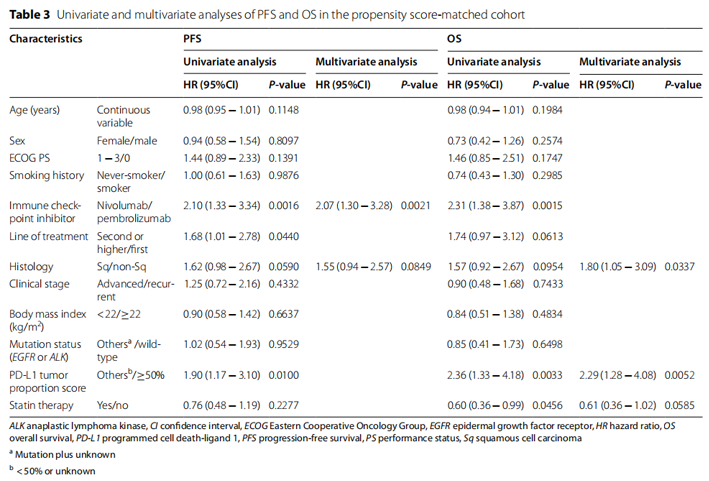
11. What is the best treatment for disease recurrence during or after adjuvant ositinib administration?
Consensus: prospective data on this clinical situation are limited. The data show that if the disease relapses after completing adjuvant ositinib, it may be effective to challenge ositinib again, and it is recommended to take ositinib again for 3 years. However, if the patient has disease recurrence when receiving adjuvant therapy with ositinib, it is recommended to stop adjuvant therapy with ositinib and conduct tissue biopsy of the recurrent lesions to guide the next treatment, because it is possible to detect the resistance mechanism of ositinib. Local ablation can be considered for the treatment of few recurrent lesions. (evidence level: I, recommendation level: a)









1. what is the best first-line treatment for patients with common EGFR mutations?
Consensus: third generation EGFR TKIs (such as ositinib) are considered as first-line treatment options for patients with advanced non-small cell lung cancer carrying common EGFR mutations. (evidence level: I, recommendation level: a)
2. What is the best treatment for patients with central nervous system (CNS) and / or leptomeningeal metastases?
Consensus: in patients with CNS metastasis (including meningeal metastasis), the third generation EGFR TKI should be given priority as the initial treatment. Prospective controlled trial data do not support EGFR TKI combined with radiotherapy. For patients with intracranial progression despite 80 mg of ositinib, local stereotactic radiotherapy should be given to avoid WBRT. Continued use of 80 mg of ositinib is the standard protocol, and the dose of ositinib can also be increased by 160 mg. In patients with meningeal metastasis, 160mg of ositinib was the preferred dose. (evidence level: II, recommendation level: a)
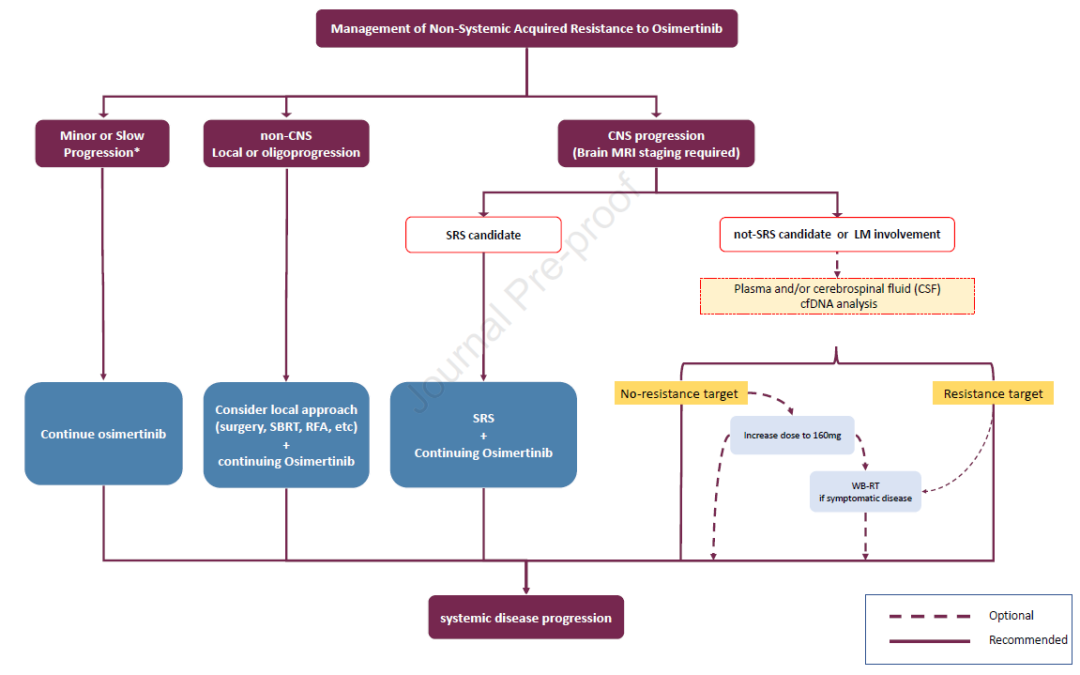
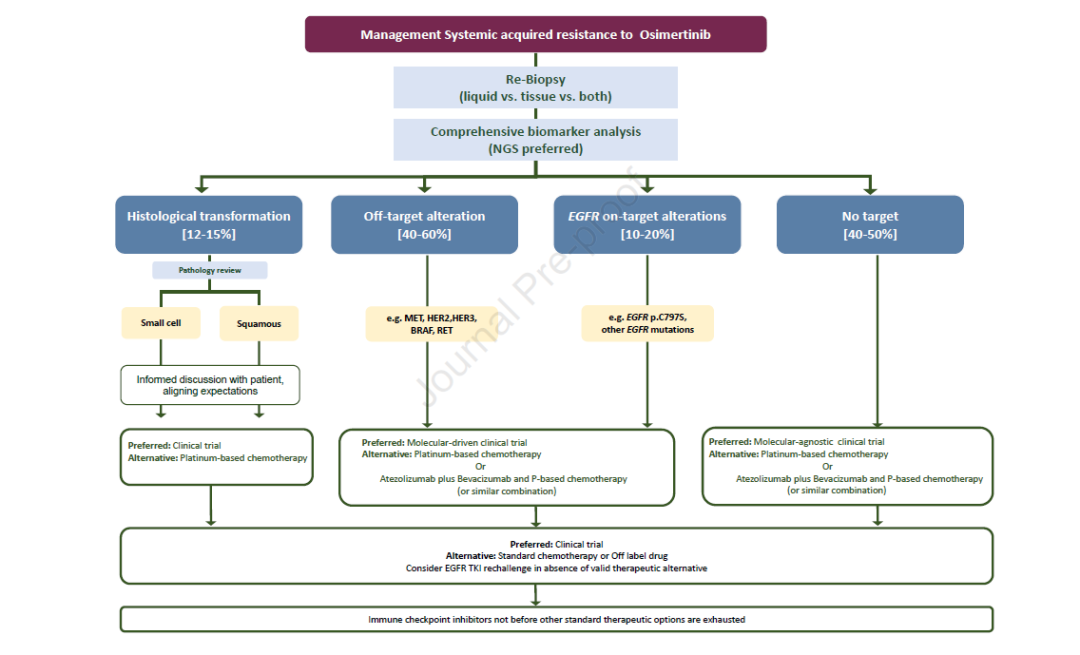
3. What is the best treatment plan after the failure of ositinib?
Subsequent management will depend on patient and disease characteristics, genetic testing results, and access to treatment or clinical trials.
3a) what is the best treatment plan for patients with EGFR resistance mutation (on target) after treatment failure of ositinib?
Consensus: in clinical practice, platinum containing chemotherapy should be regarded as standard treatment. In the context of clinical trials, when new EGFR mutations occur after treatment with ositinib, alternative targeted therapies, including TKI, monoclonal antibodies (mAbs) and antibody drug conjugates (ADCs), should be considered as the preferred treatment. (evidence level: II, recommendation level: b)
3b) what is the best treatment plan for patients with bypass activation after treatment failure with ositinib?
Consensus: the mechanisms of acquired resistance to ositinib include targetable bypass activation, such as met amplification and HER2 amplification. Although platinum containing chemotherapy is still the standard treatment in this case, treatment schemes targeting specific drug resistance mechanisms have shown efficacy, and such patients are encouraged to preferentially participate in molecular driven clinical trials. (evidence level: II, recommendation level: b)
3C) what is the best treatment plan for patients with histological type transformation after treatment failure of ositinib?
Consensus: etoposide combined with platinum based chemotherapy is recommended for patients with small cell transformation after EGFR TKI resistance. It is not clear whether ositinib needs to be continued during chemotherapy. Patients with other histological transformations should receive chemotherapy appropriate for the histological type (e.g., squamous cell carcinoma). The use of immunotherapy in this clinical setting should be evaluated in clinical trials. (evidence level: IV, recommendation level: b)
3D) what is the best treatment plan for patients without targetable changes after treatment failure of ositinib?
Consensus: for patients with slow tumor progression and no deterioration of clinical symptoms, ositinib can continue to be used until symptoms or obvious progression occur. In this case, clinical trials should be given priority. Although the optimal treatment regimen is not clear, platinum containing chemotherapy ± bevacizumab is still the standard systemic treatment regimen after resistance to ositinib.
Atilizumab + platinum containing chemotherapy + bevacizumab is also an option. The effectiveness of immune checkpoint inhibitor + platinum containing chemotherapy is not clear, and the relevant phase III randomized controlled study is ongoing. For patients with oligoprogression, including patients with CNS, continuous use of ositinib combined with local treatment (such as radiotherapy or surgery) may be considered. (evidence level: II, recommendation level: b)
4. What is the role of EGFR TKI re challenge?
Consensus: in the absence of targeted molecular driven clinical trials, for patients with disease progression (including brain metastases) who have stopped EGFR TKI for more than 6 months and have no evidence of targeting EGFR resistance mechanism, re challenge of EGFR TKI can be considered. (evidence level: III, recommendation level: C)
5. what is the role of immune checkpoint inhibitors in EGFR mutated lung cancer patients?
Consensus: regardless of PD-L1 expression, when there are other standard treatment regimens, it is not recommended to use immune checkpoint inhibitor monotherapy in patients with EGFR mutated lung cancer, because the efficacy is limited and it will increase the adverse effects of subsequent TKI treatment. Based on these facts and the potential toxicity of immunotherapy followed by ositinib; For newly diagnosed patients with advanced NSCLC, it is recommended to obtain genetic test results before immunotherapy. (evidence level: II, recommendation level: b)
6. What is the best treatment for lung cancer patients with EGFR 20 exon insertion mutation?
Consensus: platinum containing chemotherapy regimen should be used as the first-line treatment, and immune checkpoint inhibitors are not recommended to avoid potential toxicity risk in the later line targeted treatment. After platinum containing chemotherapy fails, amivantamab or mobocertinib is recommended as the second-line treatment. (evidence level: II, recommendation level: b)
7. What is the best treatment for patients with rare EGFR mutation lung cancer?
Consensus: Based on relevant data, afatinib and ositinib should be the first-line treatment for patients with rare EGFR mutations (p.g719x, p.l861q, p.s768i) or compound mutations. If targeted drugs are not available, chemotherapy may be considered. (evidence level: II, recommendation level: b)
8. What is the best treatment for patients with EGFR mutations combined with other driver mutations?
Consensus: it is recommended to use ngs comprehensive analysis to evaluate whether there are multiple driver gene mutations and determine which driver gene is dominant. If co mutation is confirmed, platinum containing chemotherapy ± TKI is preferred. (evidence level: IV, recommendation level: C)









Source link:
https://www.medsci.cn/article/show_article.do?id=5afee36795cd