Proton pump inhibitors (PPIs) are commonly used in the treatment of peptic ulcer. Among tumor patients, 20%~33% use PPIs to alleviate the symptoms of gastroesophageal reflux, while the use of PPIs in gastrointestinal cancer, pancreatic cancer, glioblastoma, gastrointestinal stromal tumors is more frequent (35%~50%). Currently, commonly used PPIs in clinic include omeprazole, rabeprazole, esomeprazole, etc.
However, numerous clinical trials and studies have concluded that the "abuse" of PPI will affect the efficacy of anti-tumor therapy. Both in chemotherapy, targeted therapy and immunotherapy. The effect of PPI on anti-tumor therapy is summarized below. The latest research shows that the use of antibiotics and PPI may be related to the poor prognosis of cancer patients receiving ICI treatment. The use of antibiotics (ATB) and proton pump inhibitors (PPI) will change the composition and diversity of intestinal microbiota, thus affecting the immune system, and thus interfering with the response against PD1 immune checkpoint inhibitors (ICI). This article published on Front Immunol will give us some inspiration. This article retrospectively included 212 patients with non-small cell lung cancer, melanoma, gastrointestinal tumor or renal cell carcinoma treated with ICI.

Patients who received ATB treatment within 60 days before the start of ICI were included in the ATB + group. Patients who received PPI within 30 days before the beginning of ICI were included in the PPI+group. The patients who finally met the criteria were divided into four groups, namely: ATB -/PPI -, ATB+/PPI -, ATB -/PPI+, ATB+/PPI+.
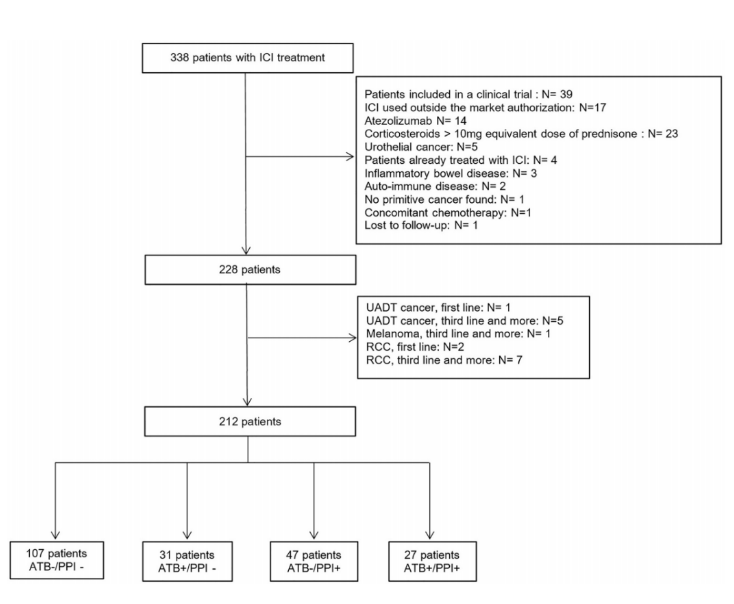
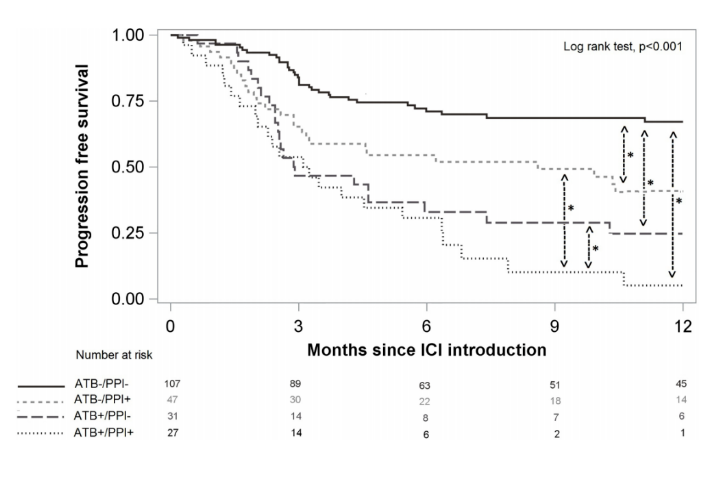
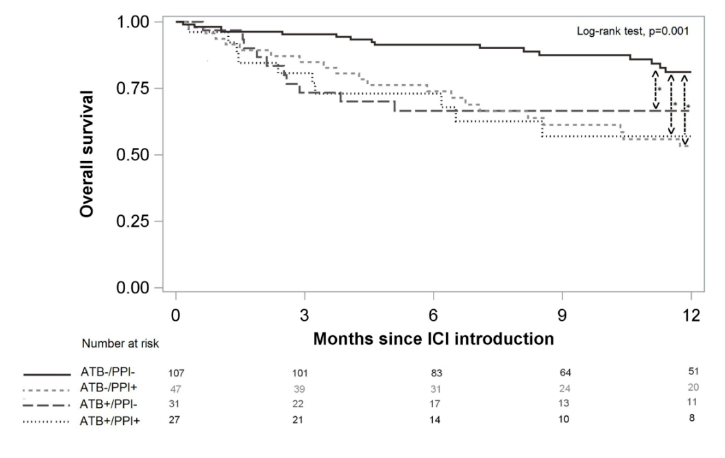
Final results: PFS was 56.7% at 6 months, 95% CI (49.6%; 63.2%), 47.2% at 12 months, 95% CI (39.8%; 54.1%). OS was 81.6%, 95%ci (75.6%; 86.2%) at 6 months and 69.4%, 95%ci (61.9%; 75.7%) at 12 months. Compared with the ATB -/PPI group, the PFS of the ATB+/PPI group was lower [hazard ratio (HR) 1.90, 95% CI (1.41; 2.57)] and the ATB -/PPI+group [HR 1.51, 95% CI (1.11; 2.05)], and the ATB+/PPI+group was the lowest [HR 3.65, 95% CI (2.75; 4.84)]. For OS, whether ATB or PPI is used alone or combined with ATB and PPI is a risk factor leading to death, and the combination of ATB and PPI will not further increase the risk. Among the adverse events observed in 78 cases (36.8%), the use of ATB or PPI had no significant effect. In an article on the efficacy of antibiotics, proton pump inhibitors and immunotherapy published on Annals Of Oncology, the retrospective analysis of the study used summary data from Phase II POPLAR (NCT 01903993) and Phase III OAK (NCT 02008227) trials, including 1512 previously treated patients with non-small cell lung cancer (NSCLC) who were randomly assigned to receive atezumab (n/757) or docetaxel (n/755). The primary objective was to assess the impact of the use of ATB and PPI on overall survival (OS) and progression free survival (PFS).
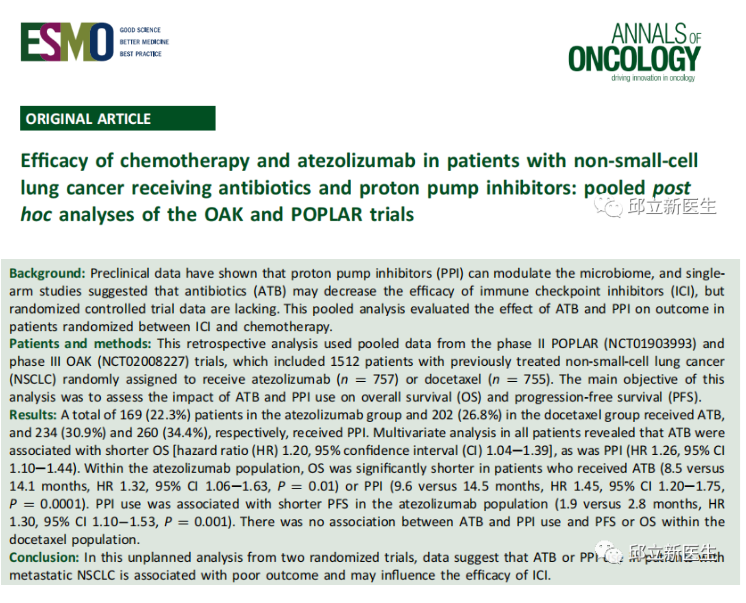
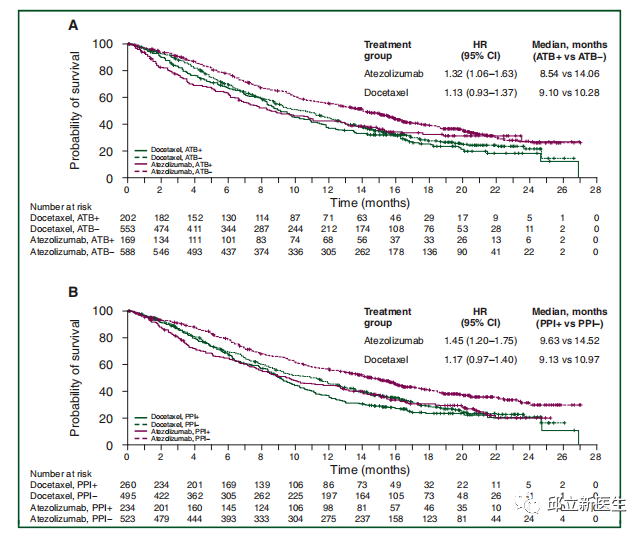
This retrospective analysis used pooled data from the phase II poplar (NCT 01903993) and phase III oak (NCT 02008227) trials, including 1512 previously treated patients with non-small cell lung cancer (NSCLC) who were randomly assigned to receive atzumab (n / 757) or docetaxel (n / 755). The primary objective was to assess the impact of the use of ATB and PPI on overall survival (OS) and progression free survival (PFS).
The results showed that 169 cases (22.3%) in the atezumab group and 202 cases (26.8%) in the docetaxel group were given ATB and PPI, respectively, 234 cases (30.9%) and 260 cases (34.4%). Multivariate analysis of all patients showed that ATB was associated with shorter OS [hazard ratio (HR) 1.20, 95% confidence interval (CI) 1.04e1.39] and PPI (HR 1.26, 95% CI 1.10e1.44). In the atezumab group, patients receiving ATB (8.5 vs 14.1 months, HR 1.32, 95% CI 1.06e1.63, P/4 0.01) or PPI (9.6 vs 14.5 months, HR 1.45, 95% CI 1.20e1.75, P/4 0.0001) had significantly shorter OS. The use of PPI was relatively short related to atezumab PFS (HR 1.30, 95% CI 1.10? 1.53, P 0.001). In the docetaxel population, the use of ATB and PPI was not associated with docetaxel. Data show that the use of ATB or PPI in patients with metastatic NSCLC is associated with poor prognosis and may affect the efficacy of ICI.
A study based on SEER database showed that for elderly cancer patients ≥ 65 years old, the proportion of proton pump inhibitors (PPIs) during TKIs treatment was as high as 22%. The risk of death within 90 days of lung cancer patients treated with erlotinib combined with PPIs increased by 21%, and the risk of death within 1 year increased by 11%.

Chu et al retrospectively analyzed the effect of acid suppressants on the efficacy of erlotinib. A total of 507 NSCLC patients were included, including 115 patients in the antacid group taking PPIs and 9 patients taking H2RA. The results showed that the progression free survival (PFS) of the combination group (acid inhibitor+erlotinib) and the single drug group (erlotinib) were 1.4 and 2.3 months (P<0.001), respectively, and the overall survival (OS) was 12.9 and 16.8 months (P=0.003), respectively.

Niho et al. conducted a retrospective study, including 117 patients with advanced NSCLC who took gefitinib 250 mg/d and were divided into acid fast group and non acid fast group for treatment. The study results showed that the median PFS of acid fast group (PPIs/H2RA) and non acid fast group were 8.7 and 10.7 months respectively (P=0.13), the objective response rate (ORR) was 64% and 63% (P=0.92), and the OS was 20.1 and 24.3 months respectively (P=0.07), There was no significant difference in ORR and PFS between the two groups, but there was a statistical difference in OS.
In addition, another study on gefitinib also suggested that the combination of PPIs treatment would increase the risk of death of patients.
In a clinical study to evaluate the impact of PPIs on the bioavailability of dacotinib, 24 healthy male volunteers were included in the study. The results showed that rabeprazole could delay the median tmax of dacotinib, and AUCinf and Cmax decreased by 71.1% and 49.5%, respectively. This indicated that when combined with rabeprazole, the blood concentration of dacotinib decreased significantly, suggesting that the combination of dacotinib and PPIs should be avoided. In the guiding principles for clinical application of new anti-tumor drugs (2020 Edition), the rational use of dacotinib, a second-generation EGFR inhibitor for the treatment of advanced EGFR mutated non-small cell lung cancer, also clearly points out: when taking dacotinib, avoid using proton pump inhibitors at the same time; Locally acting antacids or H2 receptor antagonists can be used to replace proton pump inhibitors; When H2 receptor antagonists must be taken temporarily, the product should be given at least 6 hours in advance or 10 hours later. In 2016, a study was conducted in JAMA oncology to determine whether proton pump inhibitors would reduce the efficacy of capecitabine. In this study, 545 patients with advanced Her-2 positive gastroesophageal cancer who received capecitabine and oxaliplatin (CapeOx) in combination with or without lapatinib (TRIO-013/LOGiC) were reanalyzed.

The use of proton pump inhibitors was determined through drug records, and the progression free survival (PFS) and overall survival (OS) of patients receiving PPI were compared with those without PPI. The results showed that among the 545 patients included in the analysis (median age: 60 years, 406 males), 229 patients received PPI (lapatinib, n = 110; control group, n = 119), which were equally distributed between the two groups. In patients receiving CapeOx regimen, the median PFS (4.2 months vs.5.7 months; HR=1.55; 95% CI 1.29-1.81, P<0.01) and median OS (9.2 months vs.11.3 months; HR=1.34; 95% CI, 1.06-1.62, P=0.04) in the proton pump inhibitor and non proton pump inhibitor groups were (72% vs.83%; P=0.02), and the disease control rate was (72% vs.83%; P=0.02). Multivariate analysis also showed that patients receiving PPI had shorter PFS (P<0.01).
Conclusion: Proton pump inhibitors may increase the pH of the stomach, thereby reducing the efficacy of capecitabine. However, this study did not test the pharmacokinetics and circulating drug levels, so this analysis cannot be directly related to the potential reduction of capecitabine absorption. The same phenomenon was also observed in the tumor targeted therapy of tyrosine kinase inhibitors erlotinib and sunitinib. So can tumor patients use PPI?
In order to further standardize the clinical application of proton pump inhibitors (PPIs) and promote rational drug use, the country has formulated the Guidelines for the Clinical Application of Proton Pump Inhibitors (2020 Edition). It is clearly pointed out that chemotherapy drugs and glucocorticoids can cause mucosal damage, dyspepsia and stress ulcer in patients. Proton pump inhibitors can be used to improve the symptoms of heartburn and nausea, and improve the quality of life of tumor patients during tumor chemotherapy. However, the prophylactic use of proton pump inhibitors before conventional chemotherapy is not recommended. The following guidelines also clearly define the preventive use of PPIs. In the Expert Consensus on the Preventive Use of Proton Pump Inhibitors 2018, it is pointed out that when cancer patients use chemotherapy drugs with vomiting risk intravenously, PPIs can be used intravenously for a short period of time. In 2019, the Expert Guidance on Preventive Use of Proton Pump Inhibitors and Prescription Simplification pointed out: ① Although many domestic and foreign cancer chemotherapy anti emetic guidelines mentioned that PPI can be used for the prevention and treatment of chemotherapy vomiting, based on the current evidence, it is not recommended to use PPI in conventional chemotherapy to prevent vomiting or possible upper gastrointestinal symptoms. ② If the patient is complicated with digestive tract symptoms, short-term PPI can be given symptomatic treatment, and oral dosage forms should be given as far as possible. ③ If secondary gastrointestinal bleeding occurs, therapeutic drugs should be taken under the guidance of a specialist. In May 2020, the expert consensus on the optimal application of proton pump inhibitors pointed out that the optimal suggestions for the prevention of gastric mucosal damage caused by tumor chemotherapy are: routine use of PPIs during chemotherapy is not recommended to prevent gastric mucosal damage; When cancer patients use chemotherapeutic drugs with risk of vomiting, if they have stomach discomfort, they can use PPIs in the short term in the antiemetic program until the end of chemotherapy. Based on the above evidence of evidence-based medicine, proton pump inhibitors must be used with caution during tumor treatment.
Reference
1.Giordan Q, Salleron J, Vallance C, et al. Impact of Antibiotics and Proton Pump Inhibitors on Efficacy and Tolerance of Anti-PD-1 Immune Checkpoint Inhibitors. Front Immunol. 2021 Oct 27;12:716317.
2.Pinato DJ, Howlett S, Ottaviani D, et al. Association of Prior Antibiotic Treatment With Survival and Response to Immune Checkpoint Inhibitor Therapy in Patients With Cancer. JAMA Oncol. 2019 Dec 1;5(12):1774-1778.
3.Chalabi M, Cardona A, Nagarkar DR, et al. Efficacy of chemotherapy and atezolizumab in patients with non-small-cell lung cancer receiving antibiotics and proton pump inhibitors: pooled post hoc analyses of the OAK and POPLAR trials. Ann Oncol, 2020, 31(4): 525-531.
4. Sharma M, Holmes HM, Mehta HB, et al. The concomitant use of tyrosine kinase inhibitors and proton pump inhibitors: Prevalence, predictors, and impact on survival and discontinuation of therapy in older adults with cancer. Cancer, 2019, 125(7): 1155-1162.
5. Chu MP, Ghosh S, Chambers CR, et al. Gastric acid suppression isassociated with decreased erlotinib efficacy in non–small-cell lung cancer[J]. Clinical Lung Cancer, 2015, 16(1): 33-39
6. Niho S, You Y, Zenke k, et al. Clinical impact of gastric acidsuppressing medication use on the efficacy of erlotinib and gefitinib in patients with advanced non-small-cell lung cancer harboring EGFR mutations[J] . Clin Lung Cancer, 2016, 17(5), 412-418.
7. Fang YH, Yang YH. Concurrent proton-pump inhibitors increase risk of death for lung cancer patients receiving 1st-line gefitinib treatment-a nationwide population-based study. Cancer Manag Res, 2019, 11: 8539-8546.
8. Ruiz-Garcia A, Masters JC, Laure MDC, et al. Effect of food orproton pump inhibitor treatment on the bioavailability of dacomitinib in healthy volunteers[J]. The Journal of Clinical Pharmacology, 2016, 56(2): 223-230.
9. 质子泵抑制剂临床应用指导原则(2020 年版)
10. Capecitabine Efficacy in Advanced Gastroesophageal Cancer.JAMA Oncology.June 2017
11. 《质子泵抑制剂预防性应用专家共识 2018》
12. 《预防性使用质子泵抑制剂及处方精简专家指导意见 2019》
13. 《质子泵抑制剂优化应用专家共识 2020》
Source link:
https://www.cn-healthcare.com/articlewm/20220909/content-1432752.html