Patients with malignant tumors often have nausea and vomiting during chemotherapy, which not only increases the pain of patients, greatly reduces the quality of life, makes patients have feelings of resistance and fear to chemotherapy, affects the implementation and efficacy of chemotherapy, but also affects the intake of food nutrition. In serious cases, it may even lead to dehydration, electrolyte disorders, and weight loss.
The frequency and extent of chemotherapy related nausea and vomiting (CINV) depend on the individual differences of patients and the chemotherapy program, and vary according to the type, dose, usage and route of chemotherapy drugs.
Classification of chemotherapy related nausea and vomiting
Anticipatory vomiting: It occurs in patients who have received chemotherapy, and nausea and vomiting occur before the next chemotherapy, which is often associated with unpleasant experience of previous chemotherapy.
Acute vomiting: It occurs within minutes to hours after chemotherapy. The peak usually lasts for 5 to 6 hours, and it usually relieves within 24 hours.
Delayed vomiting: it occurs 24 hours after chemotherapy, usually in patients receiving cisplatin, cyclophosphamide and anthracycline drugs.
Outbreak vomiting: refers to nausea and vomiting reaction that still occurs after preventive treatment and requires "rescue treatment" of antiemetic drugs.
Refractory vomiting: refers to the vomiting that still occurs in the subsequent chemotherapy cycle after the failure of preventive and/or rescue antiemetic treatment in the previous chemotherapy cycle.
Classification of antiemetic drugs
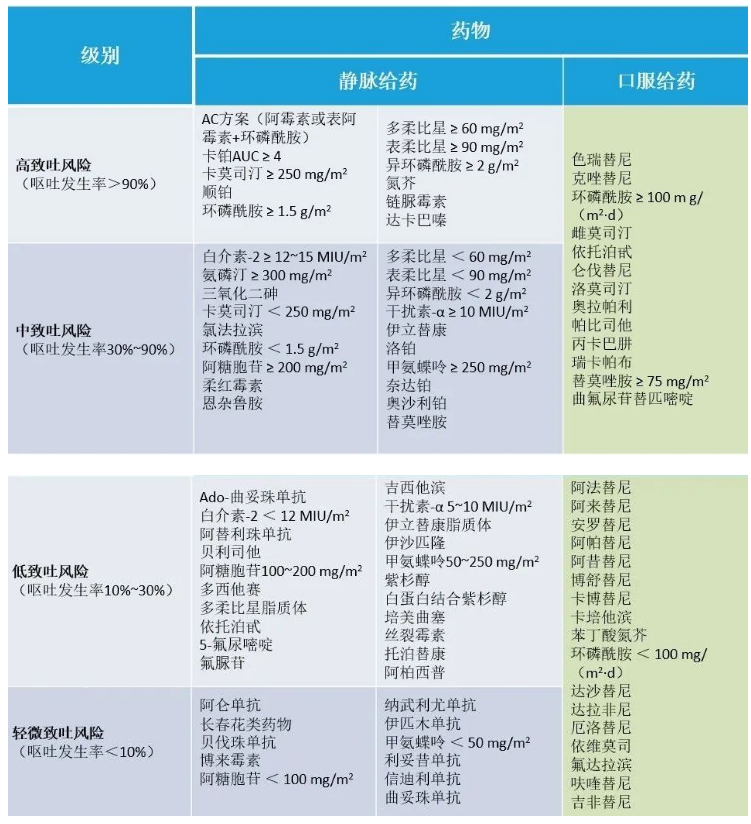
At present, the risk of vomiting caused by chemotherapy drugs is divided into four levels, namely, high risk of vomiting (HEC, ≥ 90%), medium risk of vomiting (MEC, 30%~90%), low risk of vomiting (10%~30%) and very low risk of vomiting (<10%).
Classification and mechanism of antiemetic drugs
Prevention of chemotherapy related nausea and vomiting (CINV)
Intravenous antiemetic regimen
Oral antiemetic regimen
Introduction and precautions of commonly used antiemetic drugs
1) 5-HT3 receptor antagonist
Chemotherapy can release 5-HT3 from chromaffin cells of the digestive tract and bind to 5-HT3 receptor of vagal nerve endings of the digestive tract mucosa, thus stimulating the vomiting center to cause vomiting. 5HT3 receptor antagonists play an antiemetic role by combining with 5-HT3 receptor of digestive tract mucosa. Various setron drugs have similar antiemetic effect and safety, and can be exchanged. The efficacy and safety of oral and intravenous drugs are similar. Common adverse reactions include mild headache, transient asymptomatic elevation of transaminase and constipation. Increasing the dosage of 5-HT3 receptor antagonist will not increase the efficacy, but may increase adverse reactions, even serious adverse reactions (QT interval prolongation).
Ondansetron has a half-life of about 3 hours, and the peak time of blood concentration after oral administration is 1.5 hours. It is mainly metabolized in the liver, and 44%~60% of metabolites are excreted through the kidney. In the prevention of vomiting caused by moderate to high emetic chemotherapeutic drugs, ondansetron is recommended to take orally 16-24mg or intravenously 816mg on the first day, orally 8 ng bid or 16mgqd or intravenously 816mg on the second to third days. The recommended dose for rescue treatment is 16ng orally or intravenously, once a day. The intravenous dosage of ondansetron should not exceed 16mg. High dose ondansetron may cause QT interval prolongation. In view of its heart risk, FDA issued a warning in December 2012 and stopped using its single injection 32mg dosage form.
Granisetron, a highly selective 5-HT'3 receptor antagonist, has a half-life of about 9 hours, but has a large individual difference. Most drugs are metabolized by liver microsomal enzyme P4503A in liver, 12% of prototype drugs and 47% of metabolites are excreted from urine, and the rest are excreted from feces in the form of metabolites. In the prevention of vomiting caused by moderate to high emetic chemotherapy drugs, the recommended dose of granisetron abroad is 2mg orally once a day or lmg twice a day on the first to third days, or 1mg or 0.0lmg/kg intravenously. Salvatory treatment is the same as above. The commonly used dose in China is 3mg intravenously, once a day. Granisetron transdermal patch is a new dosage form of granisetron to prevent chemotherapy related vomiting, and its effect lasts for 5 days. Granisetron 34.3mg/52cm2 (patch), releasing 3.1mg drug every 24 hours. Apply the transdermal patch to the clean, dry, intact and healthy upper arm skin 24-48 hours before chemotherapy, and it can be retained for 7 days according to the chemotherapy administration plan.
Dolasetron, its active metabolite, is further metabolized in the liver through CYP2D6 and CYP3A, and then excreted with urine and feces, with a half-life of about 8 hours. In the prevention of vomiting caused by moderate to high emetic chemotherapy drugs, the recommended dose of dorasetron is 100mg orally once a day. However, the risk of arrhythmia caused by dorasetron is high (including tip torsion ventricular tachycardia). FDA has issued a warning in December 2012 to stop using dorasetron for the treatment of vomiting caused by chemotherapy.
Tropisetron has a half-life of 7-8 hours, and 70% of it is excreted in urine as metabolites. Patients with uncontrolled hypertension should be cautious when using tropisetron, and should avoid using more than 10mg to avoid the risk of further increase of blood pressure. At present, tropisetron lacks large-scale clinical evidence to prove its clinical effectiveness, and the evidence and recommendation level of tropisetron in preventing nausea and vomiting caused by chemotherapy are not high. Recommended dose: 5mg intravenously or orally only on the first day.
Palonosetron has a half-life of about 40 hours. 50% of the metabolites are metabolized in the liver. The biological activity of the metabolites is extremely low. About 80% of the metabolites are metabolized by the kidney within 6 days. Palonosetron alone has the same effect as Dorasetron in preventing acute vomiting, but is superior to ondansetron. In the observation of 24120 hours after the end of chemotherapy, the incidence of delayed vomiting in patients receiving 0.25 mg of paronorsetron before chemotherapy was reduced by 19% and 15% respectively compared with ondansetron and dorasetron. Adults were injected 0.25mg intravenously about 30 minutes before chemotherapy, and applied on the first day. The most common adverse reactions were similar to the first generation 5-HT3 receptor antagonists.
The clearance of Ramosetron was biphasic, with a half-life of about 5 hours. The excretion rate of prototype drug in urine within 24 hours after administration was 16%~22% of the dose. Ramosetron has two dosage forms: orally disintegrating tablets of 0. lmg and injections of 0.3 mg (2m). For adults, 0.1 mg orally disintegrated and 0.3 mg intravenously administered once a day. In addition, when the effect of appropriate dosage increase or decrease is not obvious according to age and symptoms, the same dosage can be added, but the daily dosage shall not exceed 0.6 mg. Occasionally, it can cause shock anaphylactic symptoms (the incidence is not clear) and epileptic seizures.
Azasetron clearance showed a biphasic decrease, with a half-life of about 4.3 hours. About 64.3% of the prototype drug was excreted from urine within 24 hours. The common dosage for adults is 10mg intravenous injection, once a day. The elderly and those with renal insufficiency should use with caution or reduce the dosage. Due to the lack of research on drug safety for children, it is forbidden for children.
2) Glucocorticoid
Dexamethasone is a long-acting glucocorticoid with a biological half-life of 190 minutes and a tissue half-life of 3 days. 65% of the drugs are discharged from the kidney within 24 hours. Dexamethasone is administered orally or intravenously. To prevent acute vomiting caused by highly emetic chemotherapy, dexamethasone was used in combination with 5HT3 receptor antagonist and NK-1 receptor antagonist on the day of chemotherapy; To prevent delayed vomiting, dexamethasone was combined with NK-1 receptor antagonist for 3 consecutive days. To prevent acute vomiting caused by moderate emetic chemotherapy, dexamethasone combined with 5HT3 receptor antagonist was used for prevention on the day of chemotherapy; To prevent delayed vomiting, dexamethasone was administered continuously for 2 days. To prevent vomiting caused by low-grade emetic chemotherapy, dexamethasone was administered on the day of chemotherapy.
If the chemotherapy regimen or chemotherapy pretreatment regimen has included corticosteroids, the dosage of dexamethasone needs to be adjusted or not added. Since NK-1 receptor antagonist is an inhibitor of CYPA4 and dexamethasone is the substrate of CYPA4, dexamethasone also needs to be reduced when combined with NK-1 receptor antagonist. For patients at risk of serious adverse reactions with dexamethasone, long-term medication should be considered carefully.
3) NK-1 receptor antagonist
As a NK-1 receptor antagonist, aspirin can selectively bind with the NK-1 receptor in the brain to antagonize substance P. Substance P is a neurokinin located in the neurons of the central and peripheral nervous systems. It plays a role through the NK-1 receptor and is related to many inflammatory immune reactions such as vomiting, depression, pain and asthma. Fosapitam dimethyl glucosamine is the precursor of oral preparation of Arepidan, which is rapidly converted into Arepidan in vivo after injection.
Alepidan can effectively prevent delayed vomiting. The study showed that within 5 days after the application of cisplatin, the combined use of Arepidan, Ondansetron and Dexamethasone reduced the incidence of vomiting by 20% compared with Ondansetron and Dexamethasone. 80 mg of aripipitan can be repeatedly administered in the case of multiple days of chemotherapy or poor control of nausea and vomiting.
Arepidan can reach the peak concentration in blood within 4 hours after oral administration. It can pass through the blood brain barrier and is mainly metabolized in the liver. It may be related to cytochrome CYP3A4 and CYP1A2, with a half-life of 9 to 13 hours.
Arepidan is an inhibitor of CYP3A4, and it should be used with caution in combination with chemotherapy drugs that are mainly or partially metabolized by CYP3A4.
4) Dopamine receptor blockers
Metoclopramide (metoclopramide, metoclopramide, metoclopramide) is a dopamine receptor blocker. It increases the threshold of CTZ by inhibiting the dopamine receptor in the central emetic chemoreceptor zone (CYZ), and plays a strong central antiemetic effect. The onset time is 0.5~1h by oral administration, 1~3min by intravenous injection, the duration of action is 1~2h, the half-life is 4~6h, and it is excreted through the kidney.
In the prevention of vomiting caused by low-grade emetic chemotherapy drugs and rescue treatment, the recommended dose of metoclopramide is 10~40mg orally or intravenously every day, or once every 4~6 hours if necessary, for 3~4 days.
The adverse reaction is rare tension disorder, which may have sedentary restless leg syndrome. For patients receiving low-grade emetic chemotherapy, these drugs can be used alone on the first day of chemotherapy. Therefore, we must pay close attention to the dosage problem when using it. Large dosage can lead to death. When the drug dose is excessive, antihistamines or anticholinergics should be used for treatment. Note that anisodamine is not suitable for rescue of overdose of metoclopramide because it is difficult to pass through the blood brain barrier.
5) Psychotropic drugs
Psychotropic drugs can be considered for patients who cannot tolerate alepidem, 5-HT3 receptor antagonist and dexamethasone or have poor vomiting control, but they are not recommended to be used alone.
Haloperidol butylbenzene antipsychotics block dopamine receptors in the brain, mainly for antipsychotic and antianxiety effects, and also have a strong antiemetic effect. It is used for rescue treatment of nausea and vomiting caused by chemotherapy. Oral administration of 1-2 mg once every 4-6 hours, and the main adverse reaction is extrapyramidal reaction.
Olanzapine, an atypical antipsychotic drug, has affinity for a variety of receptors, including 5HT2 receptor, 5HT3 receptor, 5-HT6 receptor, dopamine receptor (D1, D2, D3, D4, D3, D), adrenaline and histamine H1 receptor. It is used for rescue treatment of nausea and vomiting caused by chemotherapy, 2.5~5mg orally, twice a day.
Lorazepam, also known as clozazepam, is an anti anxiety drug and a medium effective benzodiazepine sedative hypnotic. In the prevention of vomiting caused by low, medium and high emetic chemotherapy drugs and rescue treatment, 0.5~2mg is taken orally or intravenously or sublingually every 4-6 hours.
How to eat and drink when vomiting?
1. Give light and digestible food with high nutrition and vitamins;
2. Do not eat spicy, fried, high-fat, salty, sweet, cold, overheated and strong smelling foods, avoid alcohol and caffeinated drinks, and eat less foods rich in tryptophan, such as bananas, walnuts and eggplants.
3. Eat less and eat more. When the stomach is obviously full, reduce the amount of food in each meal.
4. Drink water (soup) regularly every day, and avoid drinking a lot of fast water.
5. Drink as little water as possible before and after eating. Eat solid and liquid food separately. Do not lie down immediately after eating.
6. In case of nausea, it tastes delicious, including hard sugar, mint sugar or lemon tea, or eating sour fruits.
7. When vomiting frequently or violently, stop eating all food and drinks until vomiting stops, and then slowly feed liquid (water, rice soup, etc.); At the same time, use antacids to prevent gastrointestinal bleeding.
8. Use antiemetic reasonably.
9. Don't rush to supplement after chemotherapy. It is not too late to start the supplement in the second and third weeks after chemotherapy, and it is more conducive to digestion and absorption.
The content of the article is organized from: Yimaitong Oncology Department, Lilac Oncology Time, Medical History
Source link:
http://www.chinamaitake.com/huishuhuachanp/2022101225618261397275.html